Figure 6.
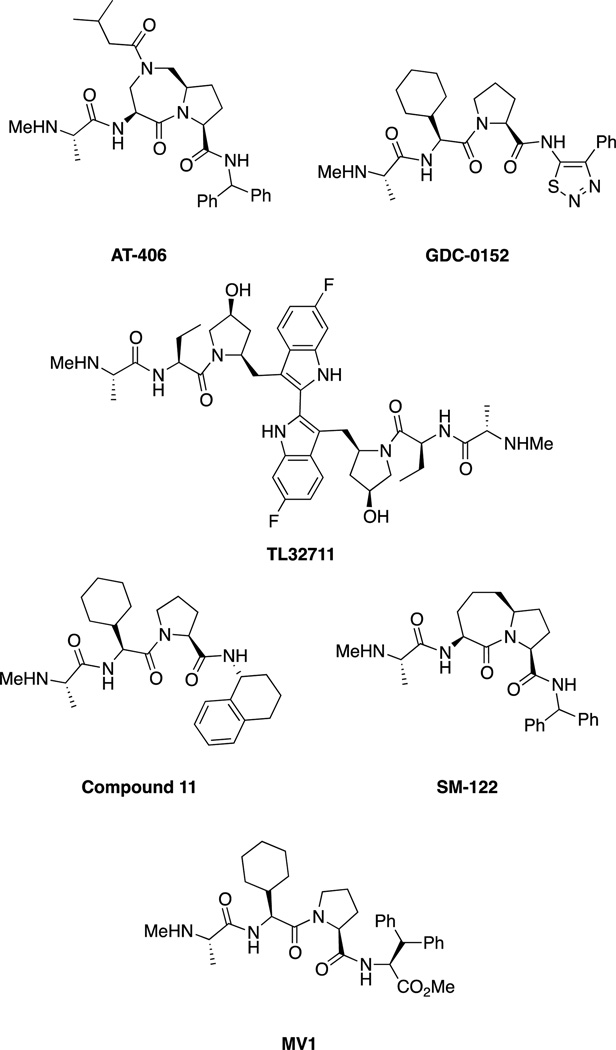
Summary of IAP inhibitors including AT-406 (developed by Ascenta Therapeutics and the University of Michigan),[62] which is administered orally in Phase 1 trials for solid tumors and lymphoma, Genentech/Roche’s GDC-0152 which is administered intravenously and is in Phase I trials for metastatic malignancies,[6b, 63] and the bivalent TL32711 (administered intravenously) developed by Tetralogics Pharma.[6b, 58] LCL161 (Novartis), AEG35156 and AEG40826 (Aegera), and YM155 (Astellas Pharma) are also in clinical trials but are not shown.[6b, 58] Selected SMAC mimics such as SM-122 and MV1 are also shown but are not in clinical trials.