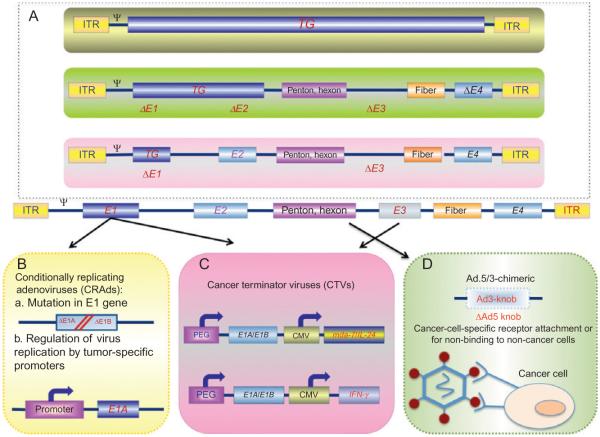
Figure 1.1.
Genetic modifications of the adenoviral genome result in oncolytic and cancer therapeutic viruses. Serotype 5 of adenovirus (Ad.5) is the most commonly used vector for gene therapy. In an effort to decrease the immune response, when delivered inside the human body, and increase cancer cell infectivity, a number of modifications in the Ad.5 genome are resulting in unique genetically modified Ad vectors (Box A). Further modifications, including mutations in the E1 region of Ad to permit cancer cell-specific replication or tumor-specific promoters driving the E1A gene, result in the development of conditionally replicating Ads (CRAds; Box B). Recombinant therapeutic Ads are being constructed in which nonessential genes of the Ad genome are deleted (e.g., E3 region) and replaced with therapeutic transgenes (e.g., mda-7/IL-24, IFN-γ). This type of “armed” oncolytic adenovirus is referred to as a cancer terminator virus (CTV; Box C). Modifications in the fiber domain of the Ad.5 genome have also been used to generate chimeric Ad.5/3 viruses (Box D). These viruses retain high infectivity in cancer cells that use the Coxsackie and adenovirus receptor (CAR) for entry of Ad.5 and also provide entry into cancer cells in a CAR-independent manner, thereby enhancing efficacy of these viruses for cancer gene therapy.