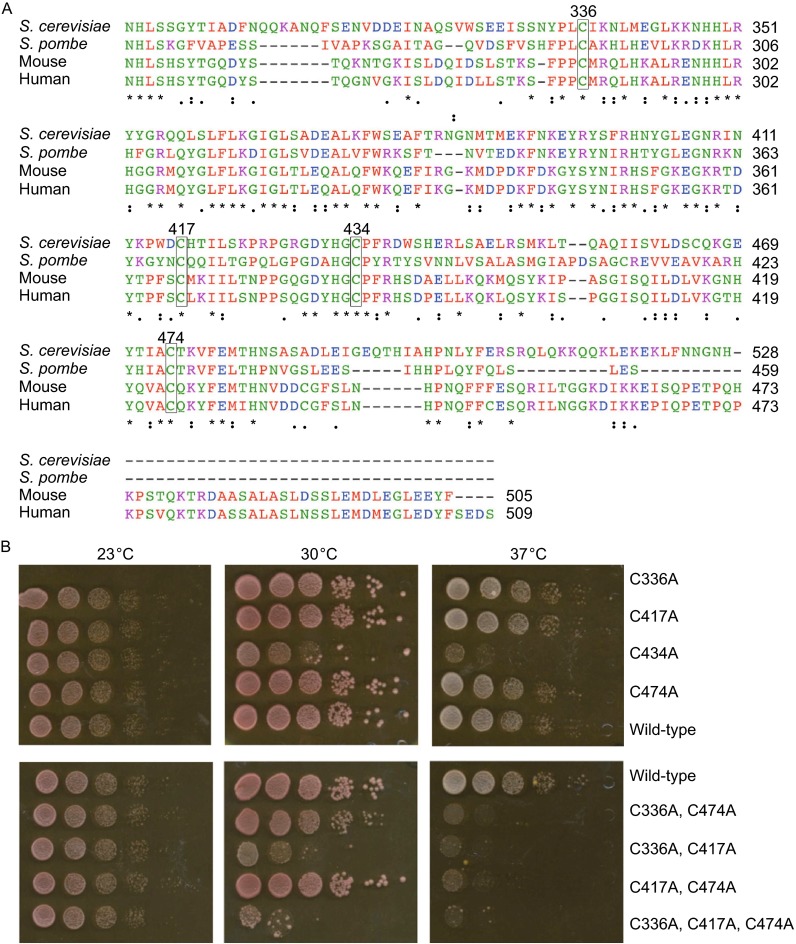
Figure 1.
Importance of conserved cysteine residues of Pri2’s C-terminal Fe-S cluster binding domain for mitotic viability. (A) Multiple sequence alignment of primase large subunit C-terminal domains. Sequences are shown for S. cerevisiae, S. pombe, H. sapiens (Human), M. musculus (Mouse). The conserved cysteines were marked in boxes. The residue numbers are those of S. cerevisiae Pri2 protein. (B) Comparison of growth between congenic wild-type and pri2 mutants with various Cys-to-Ala substitutions at different temperatures. Cells were grown in liquid YPD media to log phase (OD600 ~1) and harvested. Cell pellets were washed with water. Ten-fold serial dilutions starting at 105 cells were dot-plated on YPD plates and incubated at 23°C, 30°C and 37°C for two days before being photographed