Figure 6.
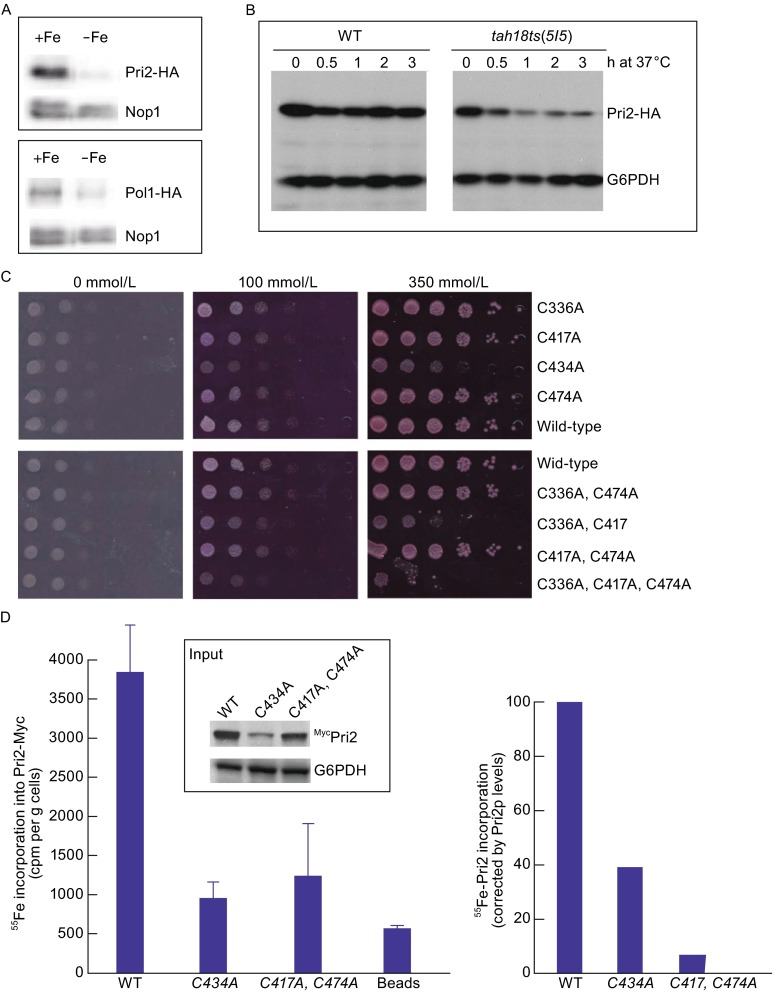
Importance of Pri2 C-terminal conserved cysteine residues for de novo iron loading into the nascent Pri2 protein. (A) Decreased Pri2 and Pol1 protein levels in cells grown under iron-deficient conditions. Wild-type cells containing integrated PRI2-HA or POL2-HA at the respective chromosomal loci were grown in regular Fe-supplemented SC media (+Fe) or in iron-depleted SC medium to log-phase before being harvested for protein extraction. Proteins were resolved by SDS-PAGE and probed with anti-HA for Pri2-HA and anti-Nop1 as a loading control. (B) Decreased Pri2 protein levels in the tah18-5I5 mutant at the restrictive temperature. Both the wild-type and tah18-5I5 ts mutant contained C-terminal triple HA epitope integrated at the chromosomal PRI2 locus. Cells were grown to log phase at 23°C before being shifted to 37°C at time zero. Protein extracts from cells collected at the indicated time points at 37°C were resolved by SDS-PAGE and probed with anti-HA for Pri2-HA and anti-G6PDH as a loading control. (C) The growth defects of pri2 cysteine mutants are independent of Fe levels in media. Congenic wild-type and various pri2 Cys-to-Ala mutants were dot-plated in 10-fold serial dilutions starting at 105 cells onto selective media containing 1 mmol/L ferrozine (maximal ferrous chelating capacity at 333 μmol/L) and supplemental ferrous ammonium sulfate at 0 μmol/L, 100 μmol/L (iron-poor) and 350 μmol/L (iron-rich). The plates were incubated at 30°C for 2 days before being photographed. (D) The Pri2 cysteine mutants are defective in iron incorporation into newly translated Pri2 polypeptide. Wild-type cells that harbor a high-copy number vector (pRS426-PTDH3) expressing the Myc-tagged wild-type (pLL98) or C434A (pLL108) and C417A, C474A (pLL112) mutant Pri2 proteins were grown in synthetic iron-poor medium supplemented with 24 μmol/L BPS for 16 h at 30°C. Radiolabeling with 55Fe was conducted for 4 h at 30°C in a BPS-free medium and Myc-Pri2 proteins were immunoprecipitated from cell extracts using an anti-Myc monoclonal antibody. The radioactivity associated with Pri2 was quantified by scintillation counting (left panel) and corrected for the differences in protein levels (right panel), which were determined by immunoblotting and quantitative densitometry (insert)
