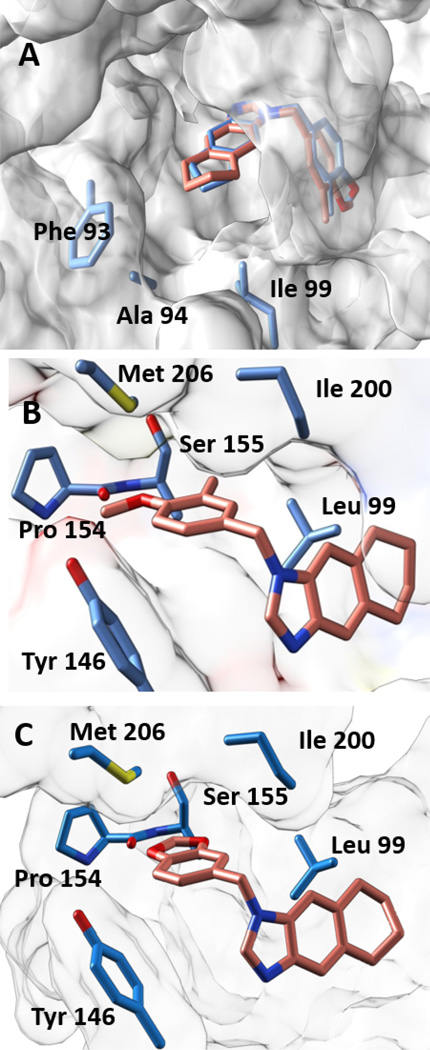
Figure 3. Co-crystal Structures.
(A) The cyclopentyl ring of 7 (blue backbone) and the cyclohexyl ring in 8 (salmon backbone) are well accommodated in the active site and face the fairly lipophilic active site opening that is lined by Phe 93, Ala94. (B) Positioning of the methyl and methoxy groups in 8 in the FtFabI active site. The methyl group at the meta position is in a very lipophilic environment. The methoxy group is surrounded by the backbone carbonyl of Pro154 (3.5 Ǻ away), the −OH of Tyr146 (4.8 Ǻ away) and −S of Met206 (3.5 Ǻ away). (C) Positioning of 10 in the active site of FtFabI. The region lined by Leu99 and Ile200 is fairly lipophilic and can accommodate fairly bulky modifications. The region lined by Met206, backbone of Pro154, and Tyr146 is more hydrophilic and restrictive but presents the possibility of exploiting hydrogen bonding in this region.