Abstract
We report a case of spondylodiscitis and spinal abscess following haematogenous dissemination of the emerging yeast Candida dubliniensis in a human immunodeficiency virus-1 (HIV-1) and hepatitis C virus (HCV)-coinfected patient. Although C. dubliniensis is considered less virulent compared to its closest known relative Candida albicans, reports of severe fungal infections are increasing. This case indicates that the pathogenicity of C. dubliniensis may be higher than previously believed. Therefore fungal infections caused by this dimorph fungus should be kept in mind in immunocompromised patients with spondylodiscitis and spinal abscess.
Keywords: Candida dubliniensis, Spondylodiscitis, Spinal abscess, Human immunodeficiency virus-1, Hepatitis C virus
1. Introduction
Originally recognized as a cause for oral candidiasis among HIV-infected patients reports of severe fungal infections caused by Candida dubliniensis are emerging in recent years [1,2].
C. dubliniensis shares many phenotypic characteristics with the predominant fungal pathogen Candida albicans like the ability of germ tube- and chlamydospore-production. Both species are able to switch between yeast- and filamentous form and are able to form biofilms on biotic and abiotic surfaces [3]. Comparison of genome sequencing show a high similarity with >80% identity [4]. Severe systemic infections by C. dubliniensis, however, have only been seen in immunocompromised patients, which indicates lower pathogenic potential of this Candida species.
Candida spondylodiscitis, often associated with significant morbidity, is rarely reported and predominantly caused by C. albicans [5]. So far only one case of spondylodiscitis caused by C. dubliniensis in an intravenous drug addict with chronic hepatitis C virus (HCV) infection has been recently reported in the medical literature [6].
2. Case
A 47-year old male was admitted with exacerbation of low-back pain radiating to the groin and the right leg to the Bernhard-Nocht-Clinic of the University Medical Center Hamburg-Eppendorf, Germany (day 0). Low-back pain and radicular symptoms started approximately one month prior to presentation with exacerbation by movement. The patient noticed no fever or chills and had no traumatic spine injury. Physical examination showed paraesthesias and a weakness of the right lower leg with a decreased patellar- and achilles tendon reflex.
He was diagnosed as HIV-1-positive 19 years ago and is currently on antiretroviral therapy with lamivudine 300 mg once daily, atazanvir 400 mg once daily and raltegravir 400 mg twice daily. At the time of presentation his CD4 T-cell count was 237/µl (normal range: 500–1350/µl) and viral load was undetectable (HIV-1 Taq-PCR: <20 copies/ml). Furthermore he was diagnosed as HCV-positive genotype 1a 13 years ago. At time of admission he presented with a HCV-related liver cirrhosis (CHILD PUGH A) without any treatment up to now and a viral load of 800 000 IU/ml. The patient had a history of intravenous drug abuse (IVDA) with cocain and benzodiazepines and is now following methadone substitution programme (methadone 120 mg once daily), any illicit drug abuse is excluded.
Results of laboratory testing showed a decreased thrombocyte count with 89×103 cells/μl (150–400×103 cells/μl) and a C-reactive protein level of 118 mg/l (reference value<5 mg/l). Basic serum and urine chemical profiles were unremarkable as well as chest radiography.
Initial magnetic resonance imaging (MRI) of the lumbar spine showed spondylodiscitis of vertebral bodies and intervertebral discs from L4 to S1 with complete destruction of L5 and contiguous epidural abscess markedly narrowing the spinal canal (Fig. 1). Due to the obstructive nature with progressive neurological impairment the abscess had to be drained twice in the first two weeks of hospitalization (day 3 and day 10).
Fig. 1.
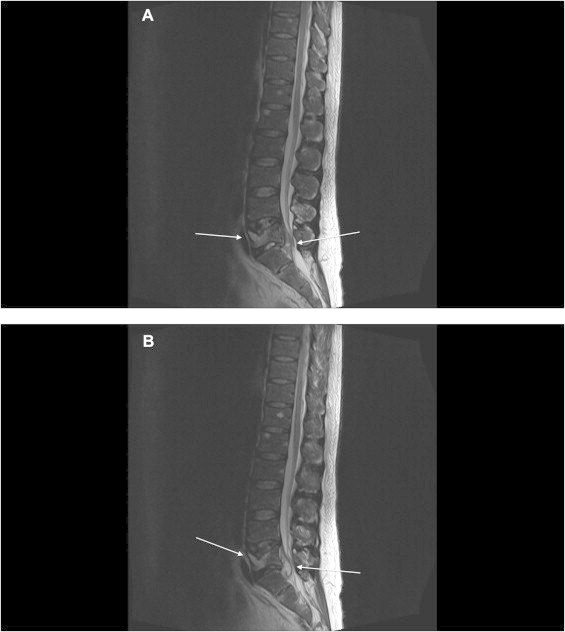
(A, B) T2 weighted MRI scans of the lumbar spine showing spondylodiscitis of vertebral bodies and intervertebral discs from L4 to S1 with complete destruction of L5 and contiguous epidural abscess markedly narrowing the spinal canal.
Culture of the abscess fluid on sabouraud dextrose agar showed white, cream-coloured colonies corresponding to Candida spp. after 24 h of incubation at 37 °C on the two occasions. The isolate was identified as C. dubliniensis by MALDI-TOF mass spectrometry (day 5). Identification was confirmed by sequencing of the ITS2 region of the ribosomal DNA with primers ITS3 and ITS4 [7] and sequence comparison to the yeast reference database at the Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre (Utrecht, Netherlands) according to Clinical Laboratory Standards Institute guideline MM18-A, (http://www.cbs.knaw.nl). The ITS2 region showed 100% sequence identity to C. dubliniensis type strain CBS7987 (GenBank accession number AB049123.1).
Furthermore three pairs of blood cultures drawn at the beginning of hospitalization (day 0) were incubated at 37 °C (BACTEC® 9240, Becton Dickinson, Heidelberg, Germany) and showed microbial growth after 26 h of incubation. Blood from positive blood culture bottles was Gram-stained and subcultured on specific agars. Further identification by MALDI-TOF mass spectrometry revealed also C. dubliniensis (day 4).
C. dubliniensis strains were tested against amphotericin B, fluconazole, voriconazole and caspofungin using E-test strips according to the manufacturer's instructions (AB Biotest, Solna, Sweden) and EUCAST guidelines [8]. The results of the susceptibility tests were: MICs of 0.064 μg/ml to amphotericin B, 0.008 μg/ml to voriconazole, 0.25 μg/ml to fluconazole and 0.125 μg/ml to caspofungin.
Candida spp. was not detectable by urine culture and fundoscopy of the eyes showed no pathological abnormalities. Transoesophageal echocardiography showed no fungal vegetation.
Antifungal treatment was initiated with fluconazole 400 mg once daily (day 4). Sultamicillin 3 g three times daily was added to cover potential co-pathogens even though bacterial pathogens were never detected in abscess or blood cultures. Sultamicillin was discontinued after 2 weeks of antiinfective treatment (day 24). Antifungal treatment was continued. As an adjunct treatment the patient had to observe strict bed rest for one month. Mobilization was achieved with a surgical corset of the affected spinal region. Under this regimen the patient stabilized clinically, no further neurological deterioration was observed. Repeat MRI after one month of treatment showed marked reduction of the abscess without any spinal stenosis and marked reduction of the inflammation in the spinal bodies (day 34).
The patient was discharged after two months. Antifungal treatment was continued for one further month with oral fluconazole 200 mg once daily.
3. Discussion
Herein, we report a severe case of spondylodiscitis and spinal abscess in a HIV-1 and HCV-coinfected patient following hematogenous dissemination of the emerging fungus C. dubliniensis.
Fungal infections caused by this dimorph fungus have been reported with increasing frequency in the medical literature. These reports include mainly endophtalmitis [9], oral candidiasis [10] and candidemia [2]. Reports of other clinical manifestations such as spondylodiscitis and osteomyelitis, however, are extremely rare. Both manifestations have been reported once previously [6,11].
Recently Oksi and colleagues described the first case of spondylodiscitis caused by C. dubliniensis in an immunocompetent intravenous drug addict (IVDA) with chronic HCV infection [6]. It remains unclear, however, if the patient had a HCV-related liver cirrhosis. This would contribute to an increased susceptibility to fungal infections as cirrhosis is considered an immunocompromised state [12]. Mortality correlated to infections is 4-times higher in patients with liver cirrhosis [13]. We suggest that liver cirrhosis played a pivotal role in our patient to develop spondylodiscitis and spinal abscess following hematogenous dissemination.
Furthermore, it remains unclear, if the patient reported by Oksi and colleagues, with a history of IVDA, was tested on HIV since an unrecognized and therefore, untreated HIV infection may compromise the immune status of the patient additionally. In our patient CD4 T-cell count was 237/µl (normal range: 500–1350/µl) at time of presentation with an undetectable viral load indicating sustaining adherence to antiretroviral therapy. Oksi and colleagues successfully treated their patient with liposomal amphotericin B for four weeks, followed by 32 weeks of fluconazole. Optimal antifungal treatment for C. dublinienis spondylodiscitis and spinal abscess remains unclear, however, suggested antifungal drugs for C. dubliniensis are comparable to C. albicans and are susceptible to antifungal drugs commonly used. In our patient antifungal susceptibility testing for amphotericin B, voriconazole, fluconazole and caspofungin showed no increased MICs. High-level resistance to azoles, however, are reported ranging from 2.5% [2] to 41% [14]. Antifungal treatment in our patient included fluconazole for 12 weeks following restitutio ad integrum.
Fungal spondylodiscitis predominantly caused by C. albicans typically involves the intervertebral disc space with narrowing of the disc cartilage, followed by destruction and lysis of the vertebral endplates and underlying vertebral bones [15]. So far, Wellinghausen and colleagues reported the only case of multifocal osteomyelitis following disseminated C. dubliniensis infection in a patient after haematopoietic stem cell transplantation (PBSCT) [11]. In line with our case, this patient reported was severely immunocompromised and showed a delayed T-cell reconstitution mediated by a lack of sufficient numbers of CD4 T-cells (<400/μl) until 6 month after the PBSCT.
This indicates that C. dubliniensis is less virulent compared to its closest known relative C. albicans, although it expresses hyphal-specific virulence factors like aspartyl proteinases Sap4 and Sap5 and the invasin ALS3, but it seems that C. dubliniensis is more susceptible to environmental stressors and hyphal formation is less efficiently compared to C. albicans [16,17]. Furthermore, C. dubliniensis triggers stronger early neutrophil responses such as neutrophil migration, phagocytosis, and the release of antimicrobial cytokines. Neutrophils internalize C. dubliniensis cells more efficiently than cells of C. albicans. The innate immunity represented by neutrophils and macrophages is believed to serve as the first line of defense by phagocytosis and direct killing of Candida. Furthermore, the antifungal immunity-related IL-17A, which is considered an important component in host defense against Candida infections, produced by peripheral blood mononuclear cells is significantly lower when challenged with C. dubliniensis in vitro [18]. Recently, Krause and colleagues even showed that candidemic patients had significantly higher IL-17A levels compared to non-candidemic patients [19].
To conclude we report the second case of spondylodiscitis and spinal abscess following hematogenous dissemination of C. dubliniensis in an immunocompromised patient. Therefore this case indicates that the pathogenicity of C. dubliniensis may be higher than previously believed. Differentiation by standard laboratory methods may be difficult and may often leads to misidentification [20]. Considering that it is likely that C. dubliniensis infections are misdiagnosed or not recognized, the burden of infection caused by C. dubliniensis may still be underestimated.
Conflict of interest
There are none.
Acknowledgements
We thank the patient for giving permission to publish the case and photographs.
References
- 1.Sullivan D.J., Westerneng T.J., Haynes K.A., Bennett D.E., Coleman D.C. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 2.Khan Z., Ahmad S., Joseph L., Chandy R. Candida dubliniensis: an appraisal of its clinical significance as a bloodstream pathogen. PLoS One. 2012;7:e32952. doi: 10.1371/journal.pone.0032952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsang P.W., Wong A.P., Yang H.P., Li N.F. Purpurin triggers caspase-independent apoptosis in Candida dubliniensis biofilms. PLoS One. 2013;8:e86032. doi: 10.1371/journal.pone.0086032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson A.P., Gamble J.A., Yeomans T., Moran G.P., Saunders D., Harris D. Comparative genomics of the fungal pathogens Candida dubliniensis and Candida albicans. Genome Res. 2009;19:2231–2244. doi: 10.1101/gr.097501.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neofytos D., Huprikar S., Reboli A., Schuster M., Azie N., Franks B. Treatment and outcomes of Candida osteomyelitis: review of 53 cases from the PATH Alliance® registry. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:135–141. doi: 10.1007/s10096-013-1939-0. [DOI] [PubMed] [Google Scholar]
- 6.Oksi J., Finnilä T., Hohenthal U., Rantakokko-Jalava K. Candida dubliniensis spondylodiscitis in an immunocompetent patient. Case report and review of the literature. Med. Mycol. Case Rep. 2014;3:4–7. doi: 10.1016/j.mmcr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White C.I., Haber J.E. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J. 1990;9:663–673. doi: 10.1002/j.1460-2075.1990.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arendrup M.C., Cuenca-Estrella M., Lass-Flörl C., Hope W., the EUCAST-AFST EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST)⁎. Clin. Microbiol. Infect. 2012;18:E246–E247. doi: 10.1111/j.1469-0691.2012.03880.x. [DOI] [PubMed] [Google Scholar]
- 9.McMillan B.D., Miller G.J., Nguyen J. Rare case of exogenous Candida dubliniensis endophthalmitis: a case report and brief review of the literature. J. Ophthalmic Inflamm. Infect. 2014;4:11. doi: 10.1186/1869-5760-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel P.K., Erlandsen J.E., Kirkpatrick W.R., Berg D.K., Westbrook S.D., Louden C. . The changing epidemiology of oropharyngeal candidiasis in patients with HIV/AIDS in the era of antiretroviral therapy. AIDS Res. Treat. 2012;2012:262471. doi: 10.1155/2012/262471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wellinghausen N., Moericke A., Bundschuh S., Friedrich W., Schulz A.S., Gatz S.A. Multifocal osteomyelitis caused by Candida dubliniensis. J. Med. Microbiol. 2009;58:386–390. doi: 10.1099/jmm.0.003970-0. [DOI] [PubMed] [Google Scholar]
- 12.Albillos A., Lario M., Alvarez-Mon M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014;6:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Arvaniti V., D’Amico G., Fede G., Manousou P., Tsochatzis E., Pleguezuelo M. Infections in patients with cirrhosis increase mortality 4-fold and should be used in determining prognosis. Gastroenterology. 2010;1256:1246–1256. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Chunchanur S.K., Nadgir S.D., Halesh L.H., Patil B.S., Kausar Y., Chandrasekhar M.R. Detection and antifungal susceptibility testing of oral Candida dubliniensis from human immunodeficiency virus-infected patients. Indian J. Pathol. Microbiol. 2009;52:501–504. doi: 10.4103/0377-4929.56138. [DOI] [PubMed] [Google Scholar]
- 15.Gathe J.C., Harris R.L., Garland B., Bradshaw M.W., Williams T.W. Candida osteomyelitis: report of five cases and review of literature. Am. J. Med. 1987;82:927–937. doi: 10.1016/0002-9343(87)90154-9. [DOI] [PubMed] [Google Scholar]
- 16.Enjalbert B., Moran G.P., Vaughan C., Yeomans T., Maccallum D.M., Quinn J. Genome-wide gene expression profiling and a forward genetic screen show that differential expression of the sodium ion transporter Ena21 contributes to the differential tolerance of Candida albicans and Candida dubliniensis to osmotic stress. Mol. Microbiol. 2009;72:216–228. doi: 10.1111/j.1365-2958.2009.06640.x. [DOI] [PubMed] [Google Scholar]
- 17.Stokes C., Moran G.P., Spiering M.J., Cole G.T., Coleman D.C., Sullivan D.J. Lower filamentation rates of Candida dubliniensis contribute to its lower virulence in comparison with Candida albicans. Fungal Genet. Biol. 2007;44:920–931. doi: 10.1016/j.fgb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Svobodová E., Staib P., Losse J., Hennicke F., Barz D., Jozsi M. Differential interaction of the two related fungal species Candida albicans and Candida dubliniensis with human neutrophils. J. Immunol. 2012;189:2502–2511. doi: 10.4049/jimmunol.1200185. [DOI] [PubMed] [Google Scholar]
- 19.Krause R., Zollner-Schwetz I., Salzer H.J., Valentin T., Rabensteiner J., Prüller F. Elevated levels of interleukin 17A and kynurenine in candidemic patients, compared with levels in noncandidemic patients in the intensive care unit and those in healthy controls. J. Infect. Dis. 2014;3:445–451. doi: 10.1093/infdis/jiu468. [DOI] [PubMed] [Google Scholar]
- 20.Ells R., Kock J.L., Pohl C.H. Candida albicans or Candida dubliniensis? Mycoses. 2011;54:1–16. doi: 10.1111/j.1439-0507.2009.01759.x. [DOI] [PubMed] [Google Scholar]


