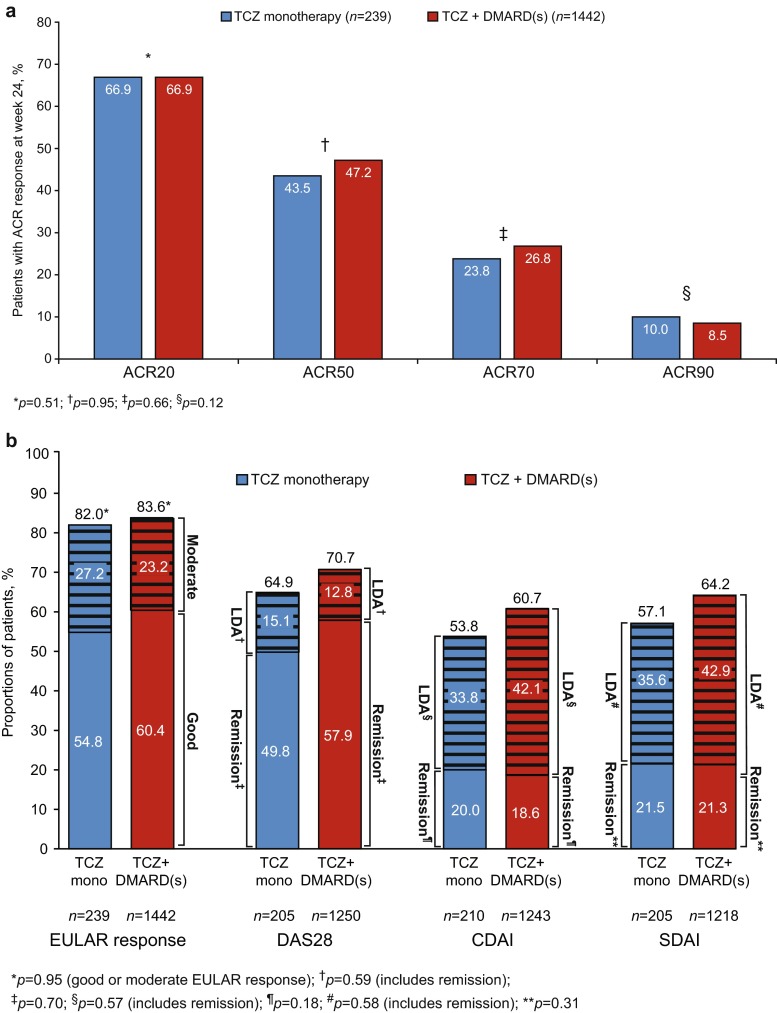
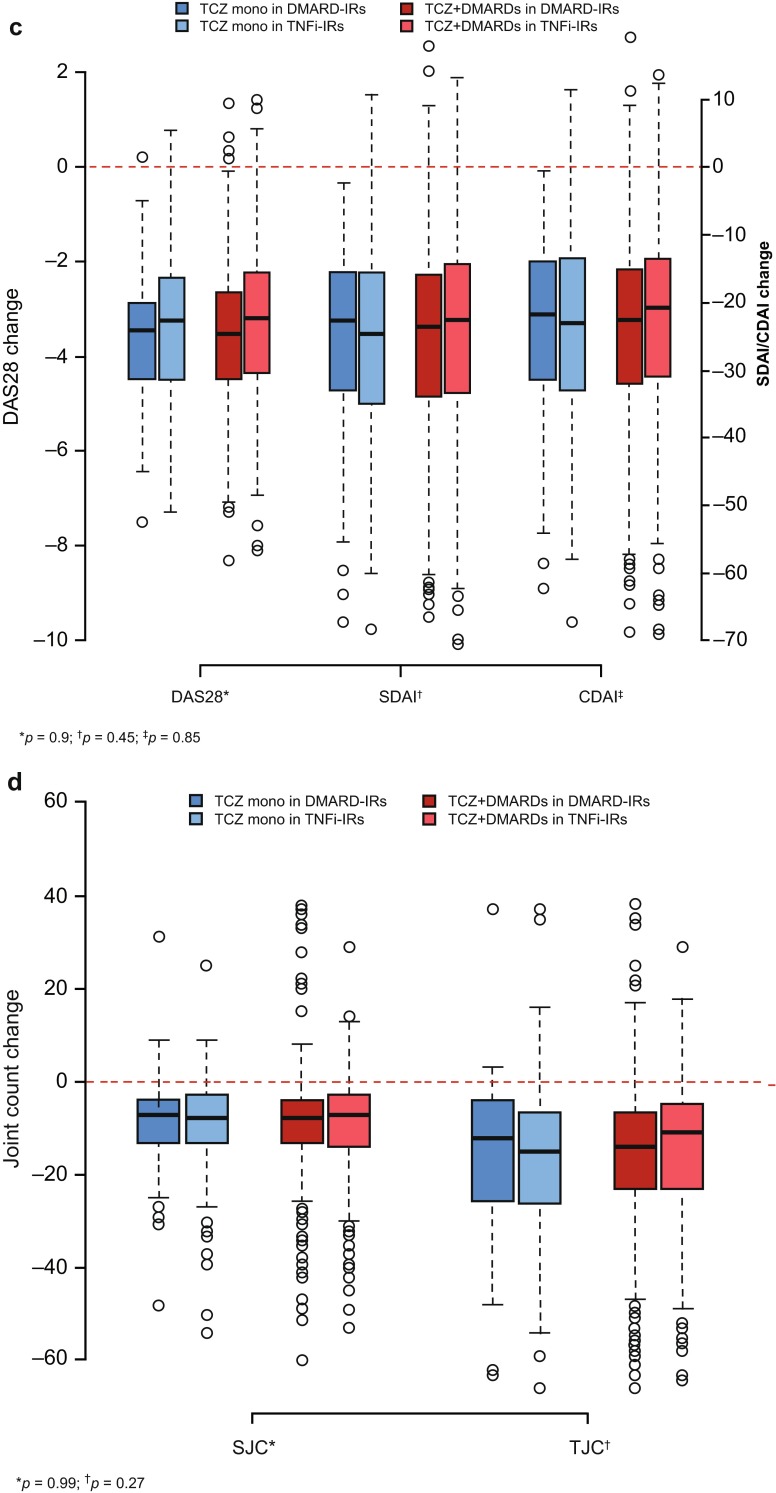
Fig. 1.
Effectiveness at week 24. a ACR20/50/70/90 responses. b DAS28, CDAI, and SDAI responses. c Change from baseline in DAS28, CDAI, and SDAI. d Change from baseline in swollen joint count (SJC) and tender joint count (TJC). a p values were calculated by logistic regression analysis adjusted for previous treatment (DMARD-IR/TNFi-IR [previous TNFi use/recent TNFi use]) and baseline DAS28. Nonresponder imputation was performed for patients who withdrew or for whom responses were missing. b Hatched lines represent moderate EULAR response or low disease activity. EULAR good response: DAS28 ≤ 3.2 at week 24 and change of >−1.2. EULAR moderate response: DAS28 ≤ 3.2 at week 24 and change of <−0.6 to ≥−1.2 or <−1.2; DAS28 > 3.2 to ≤5.1 at week 24 and change of <1.2. DAS28: low disease activity (LDA), ≥2.6 to 3.2; remission, <2.6. CDAI: LDA, >2.8 to ≤10; remission, ≤2.8. SDAI: LDA, >3.3 to ≤11; remission, ≤3.3. p values calculated by logistic regression analysis adjusted for previous treatment (DMARD-IR/TNFi-IR [previous TNFi use/recent TNFi use]) and baseline DAS28, CDAI, or SDAI, as applicable. c TCZ monotherapy in DMARD-IR patients, n = 66; TCZ monotherapy in TNFi-IR patients, n = 173; TCZ + DMARDs in DMARD-IR patients, n = 910; TCZ + DMARDs in TNFi-IR patients, n = 532. p values were calculated by Wilcoxon rank-sum test and compare TCZ monotherapy and TCZ combination therapy (disregarding the DMARD-IR-TNFi-IR split). d TCZ monotherapy in DMARD-IR patients, n = 66; TCZ monotherapy in TNFi-IR patients, n = 173; TCZ + DMARDs in DMARD-IR patients, n = 910; TCZ + DMARDs in TNFi-IR patients, n = 532; p values were calculated by Wilcoxon rank-sum test and compare TCZ monotherapy and TCZ combination therapy (disregarding the DMARD-IR-TNFi-IR split)