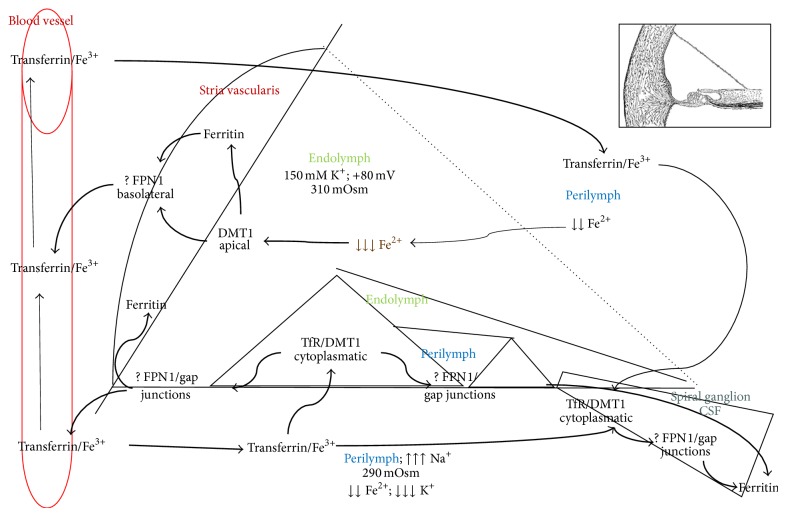
Figure 3.
Divalent metal ion homeostasis in the inner ear. The picture shows a schematic representation of a section of the inner ear; reviewing the literature allowed the speculation around the potential involvement of specific proteins in the iron homeostasis. Considering distribution and function of those proteins it may be possible that iron ions follow a cycle similar to the potassium cycle, also through gap junctions. In particular, transferrin is concentrated in perilymph and cerebrospinal fluid and it transports trivalent (ferric) ions from and to blood vessels. In addition iron ions are stocked in the stria vascularis (ferritin depots). In fact, the apical expression of DMT1 is reasonably involved in reabsorption of free divalent ions from inner ear fluids thus maintaining electrochemical features. Cytoplasmic DMT1 allows the uptake of molecules of transferrin through Tf receptors. Ferroportin (FPN 1) is basolaterally located and probably helps inner ear cells in the reuptake of divalent ions, taking them away from the sensorineural epithelium during acoustic stimulation to avoid free radical damage. Ions are taken off through the basolateral part of cells and “pushed” towards spiral ganglion and stria vascularis. It is possible that divalent ions pass through gap junctions (metal divalent ion diameter could be less than 1 nanometer; thus molecules can passively diffuse through connexons in the intercellular space). Summarizing, divalent ions are transported from area with few blood vessels and low ferritin concentrations, to the structures that can storage or evacuate divalent ions, such as stria vascularis. Outer and inner ear cells may introduce a small amount of divalent ions through calcium channels.