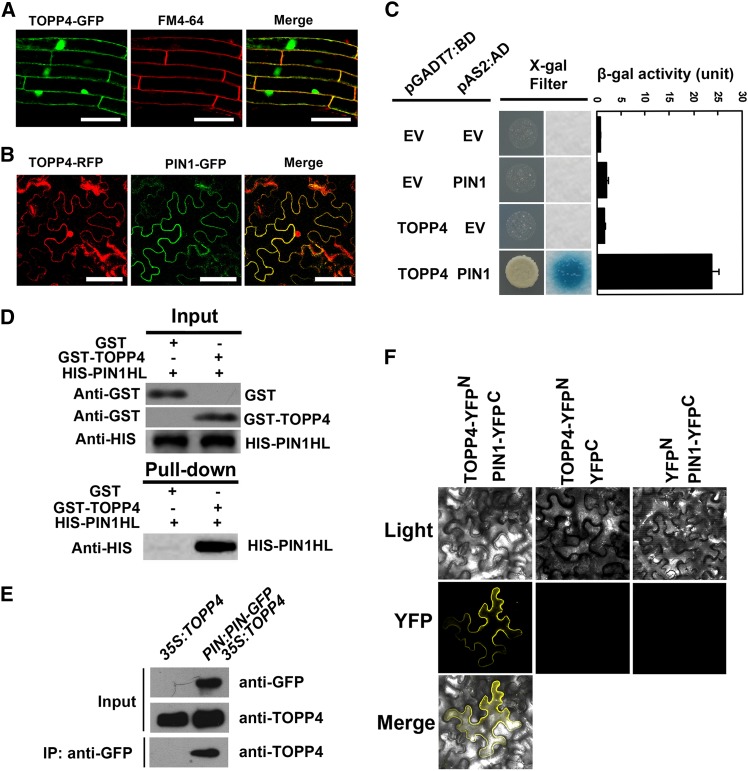
Figure 6.
TOPP4 physically interacts with PIN1 protein in vitro and in vivo. A, Subcellular localization of TOPP4-GFP and colocalization of TOPP4-GFP and FM4-64 in Arabidopsis roots. Bars = 100 µm. B, Colocalization of TOPP4-YFP and PIN1-GFP at the plasma membrane of N. benthamiana leaf epidermal cells. Bars = 100 µm. C, Yeast two-hybrid assay was used to determine the interactions between TOPP4 and PIN1. Quantitative measurements of β-gal activities are shown on the right. Error bars represent se (n = 3). X-Gal, 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside acid. D, Pull-down assay between GST-TOPP4 and HIS-PIN1. Precipitated HIS-PIN1 was detected by anti-HIS antibody. E, co-IP of TOPP4 with PIN1-GFP. Membrane extracts of PIN1:PIN1-GFP 35S:TOPP4 plants were immunoprecipitated with an anti-GFP antibody and detected by immunoblotting using an anti-TOPP4. Immunoprecipitation (IP) by the anti- GFP in 35S:TOPP4 was used as a negative control. F, BiFC assays showed that TOPP4 interacts with PIN1 at plasma membrane. TOPP4-YFPN and PIN1-YFPC fusion proteins were expressed in N. benthamiana epidermal cells. No YFP signal was detected in negative controls in which either TOPP4-YFPN or PIN1-YFPC was coexpressed with the corresponding empty vector (EV). Light indicates bright field, YFP indicates YFP fluorescence, and merge indicates merged view of the light and YFP images.