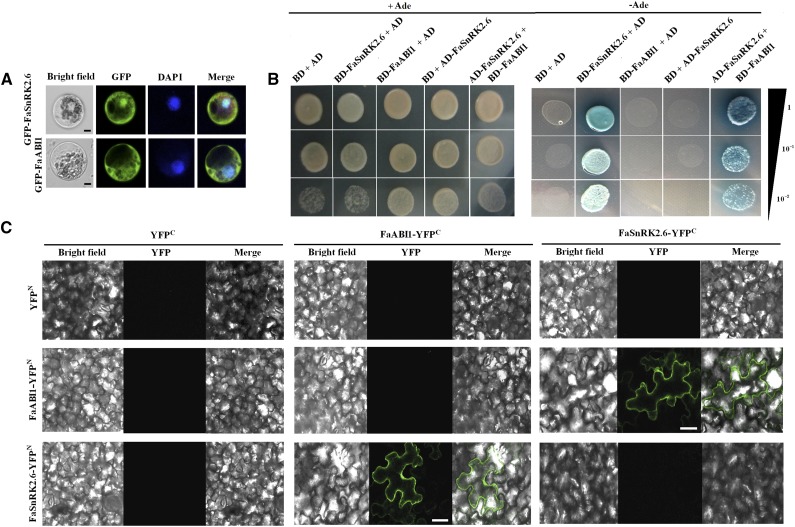
Figure 6.
Subcellular localization of FaSnRK2.6 and FaABI1 and physical interaction between FaSnRK2.6 and FaABI1. A, Subcellular localization of FaSnRK2.6 and FaABI1. pEZS-NL-Fasnrk2.6 and pEZS-NL-Faabi1 constructs were transformed into maize protoplasts, and fluorescence was observed by confocal microscopy as described in “Materials and Methods.” Bars = 8 µm. B, Yeast two-hybrid analysis of the physical interaction between FaSnRK2.6 and FaABI1. The protein interaction was examined using various combinations of prey and bait vectors. All tests were conducted on media containing adenine (+Ade; /−Leu/−Trp/+His/+Ade) or lacking adenine (−Ade; /−Leu/−Trp/−His/−Ade). Interactions were determined based on cell growth and were confirmed by an α-Gal assay on medium lacking adenine (/−Leu/−Trp/−His/−Ade). Dilutions (1, 10−1, and 10−2) of saturated cultures were spotted onto the plates. C, BiFC analysis of the physical interaction between FaSnRK2.6 and FaABI1. FaSnRK2.6 and FaABI1 were fused with either the C or N terminus of yellow fluorescent protein (YFP; designated as YFPc or YFPN, respectively). Different combinations of the fused constructs were cotransformed into tobacco (Nicotiana tabacum) cells, and the cells were then visualized using confocal microscopy as described in “Materials and Methods.” YFP and bright field were excited at 488 and 543 nm, respectively. Bars = 20 μm.