Abstract
Aurora-B is a major kinase responsible for appropriate mitotic progression. Elevated expression of Aurora-B has been frequently associated with several types of cancer, including breast cancer. However, it is not clear whether the alteration contributes to tumor responses to therapies and prognosis. In this study, we conducted immunohistochemistry using antibodies against Aurora-B, S1981p-ATM, Ki67, and p53 in paraffin-embedded tumor tissues from 312 invasive breast cancer patients. The correlation between disease-free-survival (DFS) and Aurora-B expression was analyzed using the Kaplan-Meier method and log-rank test. A Cox proportional hazards regression analysis was used to determine whether Aurora-B was an independent prognostic factor for breast cancer. We found that Aurora-B expression was correlated with the proliferation index (P < 0.001) and p53 expression (P = 0.014) in breast cancer tissues. Further we found that Aurora-B expression was associated with lymph node metastasis (P = 0.002) and histological grade (P = 0.001). Multivariate analyses indicated that elevated Aurora-B expression predicted a poor survival. In a subgroup of patients that received neoadjuvant chemotherapy, we found that elevated Aurora-B contributed to chemoresistance (P = 0.011). In conclusion, elevated Aurora-B expression in breast cancer patients contributes to chemoresistance and predicts poor prognosis.
Keywords: Aurora-B, chemoresistance, prognosis, breast cancer
Introduction
Signal transduction pathways involving in the cell cycle are typically aberrantly regulated in breast cancer. Among them is the Aurora-B kinase, a critical mitotic kinase required for the chromosome segregation, the spindle assembly checkpoint and cytokinesis [1]. Aurora-B is a primary kinase required for Serine 10 phosphorylation of histone H3 [2]. Aurora-B has been shown to regulate many of the genome stability maintenance proteins. For example, recent studies have shown that Aurora-B phosphorylates p53 on several residues (Ser183, Thr211, and Ser215) to accelerate the degradation of p53 through the polyubiquitination-proteasome pathway [3]. Aurora-B is also required for the mitotic activation of the ATM kinase, an essential kinase required for the DNA damage response [4]. Aurora-B phosphorylation of ATM on serine 1403 leads to mitotic ATM activation and required for ATM to send signals to Bub1 and Mad1 in the spindle checkpoint [4-6]. These regulatory mechanisms involving Aurora-B indicate the functional significance of Aurora-B in the maintenance of genomic and chromosomal stability. However, Aurora-B overexpression has been shown in a variety of breast cancer cell lines [7] and tumor tissues [8], suggesting there is an aberrant regulatory system in Aurora-B expression in breast cancer. The fact that overexpression of Aurora-B has been implicated in tumor formation in pre-clinical studies indicates gain-of-function when overexpressed. Despite its role in breast cancer progression, clinical implications of Aurora-B overexpression in breast cancer patients is less clear as controversial data have debated the role of elevated expression of Aurora-B and its related kinase Aurora-A in predicting survival [9,10].
To further investigate the impact of Aurora-B expression in breast cancer patients, we assessed 312 invasive breast cancer patients regarding expression of Aurora-B, ATM Serine 1981, p53 and Ki-67 (one of the important proliferation index). Interestingly we find that Aurora-B expression is correlated with poor survival and that elevated Aurora-B is associated with chemoresistance.
Materials and methods
Human breast cancer tissue samples
Paraffin-embedded materials from 312 invasive breast cancer patients with operable breast cancer treated in 2005-2006 was provided by the Department of Breast Cancer Pathology and Research Laboratory, Tianjin Medical University Cancer Hospital, Tianjin, China, with the approval of the Tianjin Medical University Institutional Review Board, and informed consent had been obtained. Clinical tumor sizes and nodal status were determined before treatment by physical examination (with or without axillary ultrasound) and with diagnostic fine-needle aspiration as required. In these cases, there were 70 patients (clinical stage II-III) who were treated with preoperative neoadjuvant chemotherapy containing sequential taxane and anthracycline-based regimens. The chemotherapeutic response was evaluated by the following standard: excellent response (pathologically complete response or minimal residual cancer burden [RCB-I]), or lesser response (moderate or extensive residual cancer burden [RCBII/III]) [11,12].
All cases were female, 26-80 years of age (mean age 52.1 years). There were 176 cases (56.4%) were involved in lymph node metastasis. The follow-up time was 14-82 (mean 63.91) months. All patients were treated either with the modified radical mastectomy (n = 186) or breast-conserving therapy (n = 126).
Pathology
The post-surgical size of the tumor was measured in fresh specimens. Tumors were cut into 0.5-cm slices, fixed in 4% buffered formaldehyde, and embedded in paraffin. Paraffin sections were cut into highly standardized 4-μm sections for hematoxylin-eosin (H&E) and immunostaining. Grade and TNM stage were assessed according to World Health Organization criteria.
Antibodies
Antibodies against Aurora-B, S1981p-ATM, Ki67, p53, Estrogen Receptor (ER) and Progestogen Receptor (PR) were purchased from Abcam (Cambridge, MA, USA). All horseradish-peroxidase conjugated secondary antibodies were obtained from Santa Cruz biotechnology.
Immunohistochemistry
Immunohistochemistry using the avidin-biotin-immunoperoxidase technique was performed for Aurora-B, S1981p-ATM, Ki67, p53, ER and PR in the 312 cases of clinical samples. Sections of formalin-fixed tissues from all cases were performed using a standard protocol. Briefly, 4 μm tissue sections on coated slides were heated for antigen retrieval, pretreated with a 3% solution of hydrogen peroxide for 5-10 minutes, rinsed and incubated with 10% normal goat serum as a blocking agent. The sections were then incubated sequentially with the primary antibodies, a biotinylated secondary antibody and avidin-peroxidase conjugate. All steps were preceded by rinsing of sections with PBS (pH 7.6). The chromogen was 3, 3’-diaminobenzidine (DAB). The sections were counterstained with hematoxylin, dehydrated and mounted. The immune-reaction for Aurora-B, S1981p-ATM, Ki67, p53, ER and PR in the nuclei of tumor cells was evaluated independently by two experienced pathologists. For the expression of Aurora-B, nuclei with fine granular staining were counted, and (-/+) was < 5% of the cells stained, (++) was 5-10% of the cells stained, and (+++) was > 10% of the cells stained. ER and PR was determined positive if finding of ≥ 1% of tumor cell nuclei were immunoreactive. S1981p-ATM, Ki67 and p53 were scored as previously described: (-) = no positive cells, (+) = 1-10% of the cells stained, (++) = 11-50% of the cells stained, and (+++) = 51-100% of the cells stained [13].
Antigen retrieval and antibody dilution were optimized prior to the study onset. To ensure uniform handling of samples, all sections were processed simultaneously. Histology was mounted onto Superfrost Plus slides (Sigma) for immuno-histochemical analysis.
Survival analysis
Patients were censored from the date of the last follow-up visit for death from causes other than breast cancer, local or regional recurrences, or the development of a second, primary cancer, including contralateral breast cancer. If a patient’s status during follow-up indicated a confirmed metastasis without a recurrence date, the follow-up visit date was used. Age, time to first recurrence, and survival time were calculated relative to the primary diagnosis date. Kaplan-Meier survival curves were constructed, and between-group differences were tested using the log-rank test. The relative importance of potential prognostic variables was tested using Cox-proportional hazard analysis and expressed with a 95% confidence interval (CI).
Statistical analysis
Data were analyzed by Student’s t test and Pearson Correlation test. Patients’ follow-up data were acquired, and survival duration was calculated from the date of diagnosis to the date of progression or last follow-up in December 2013. The correlation between disease-free-survival (DFS) and Aurora-B expression was analyzed using the Kaplan-Meier method and log-rank test. A Cox proportional hazards regression analysis was used to determine whether Aurora-B was an independent prognostic factor for breast cancer. The statistical analysis was performed with the use of software packages SPSS version 16.0 (WPSS Ltd., Surrey, United Kingdom). All statistical tests were two-sided, and a P value of < 0.05 was considered significant.
Results
Elevated expression of Aurora B is correlated with the proliferation index and p53 expression in breast cancer tissues
To investigate the clinical significance of Aurora-B expression, we conducted immunohistochemistry in 312 invasive breast cancer patients using antibodies recognizing Aurora-B, Ki-67, p53 and the activated form of ATM (ATM-S1981p) (Figure 1). These antibodies were pre-screened and selected to ensure specificity. The Pearson correlation test was used to analyze the semi-quantitative data. We found that Aurora-B expression showed a statistically significant correlation (P < 0.001) with that of Ki-67, an important proliferation marker (Table 1). However, there is no correlation with the expression of ATM-S1981p. In contrast, Aurora-B showed a strong correlation with the p53 expression level with the p-value of 0.014 (Table 2).
Figure 1.
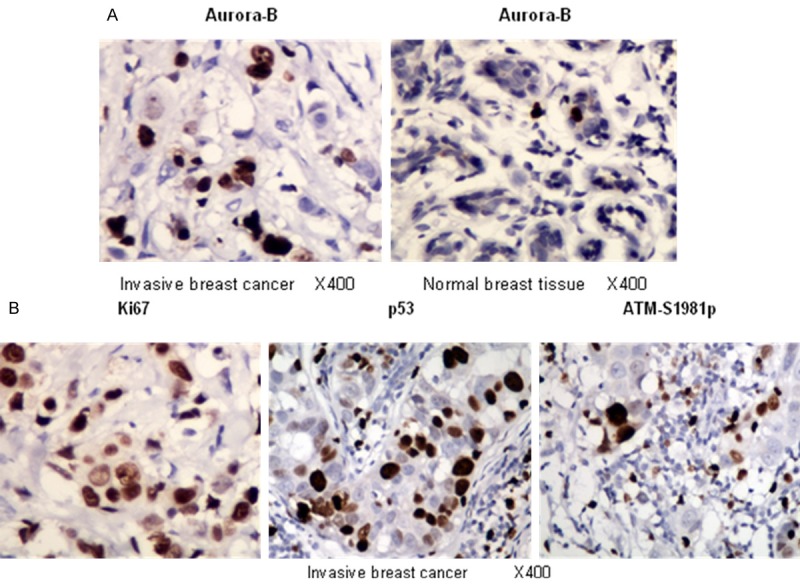
Immunohistochemical study revealing representative images of invasive breast cancer and normal breast tissues with antibody of Aurora-B, nuclei with fine granular staining were counted, and (-/+) was < 5% of the cells stained, (++) was 5-10% of the cells stained, and (+++) was > 10% of the cells stained (A); and representative images of invasive breast cancer tissues stained with antibodies of Ki67, p53 and ATM-S1981p, they were scored as (-) = no positive cells, (+) = 1-10% of the cells stained, (++) = 11-50% of the cells stained, and (+++) = 51-100% of the cells stained as described previously [13] (B).
Table 1.
Correlations of expression of Aurora B, ATM-S1981p with Ki67
| Ki67 | * P | ||||
|---|---|---|---|---|---|
|
|
|||||
| -/+ | ++ | +++ | |||
| Aurora B | -/+ | 35 | 37 | 9 | < 0.001 |
| ++ | 24 | 51 | 49 | ||
| +++ | 23 | 35 | 49 | ||
| ATM-S1981p | -/+ | 27 | 33 | 32 | 0.460 |
| ++ | 23 | 47 | 45 | ||
| +++ | 32 | 43 | 30 | ||
Pearson Correlation Test.
Table 2.
Correlation of expression of p53 with Aurora B
| p53 | *P | ||||
|---|---|---|---|---|---|
|
|
|||||
| -/+ | ++ | +++ | |||
| Aurora B | -/+ | 25 | 44 | 12 | 0.014 |
| ++ | 28 | 57 | 39 | ||
| +++ | 29 | 36 | 42 | ||
Pearson Correlation Test.
Aurora-B expression and clinicopathologic features
We then analyzed the expression pattern of Aurora-B with several clinicopathologic parameters in the tissues. We found that Aurora-B expression strongly correlated with the histological grade (P = 0.001) and status of lymph node metastasis (P = 0.002) (Table 3). However, ATM-S1981p expression showed a different correlation pattern as it only correlated with the lymph node metastasis (P = 0.001). This is consistent with our previously findings that ATM is hyperactive in breast cancer tissues with lymph node metastasis [13]. Unlike the total ATM expression pattern we tested before [14], neither Aurora-B nor ATM-S1981p expression showed a statistically significant correlation with the ER status.
Table 3.
Correlations of expression of Aurora B, p53 and S1981p-ATM with clinico-pathologic features
| Aurora B | * P | p53 | * P | S1981p-ATM | * P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| -/+ | ++ | +++ | -/+ | ++ | +++ | -/+ | ++ | +++ | |||||
| Tumor Size (cm) | ≤ 2 | 37 | 37 | 44 | 0.174 | 31 | 55 | 32 | 0.359 | 31 | 45 | 42 | 0.606 |
| 2-5 | 25 | 45 | 32 | 27 | 48 | 27 | 35 | 34 | 33 | ||||
| > 5 | 19 | 42 | 31 | 24 | 34 | 34 | 26 | 36 | 30 | ||||
| Nodal status | - | 48 | 50 | 38 | 0.002 | 38 | 70 | 28 | 0.006 | 55 | 40 | 41 | 0.001 |
| + | 33 | 74 | 69 | 44 | 67 | 65 | 37 | 75 | 64 | ||||
| TNM stage | I | 21 | 22 | 21 | 0.165 | 17 | 32 | 15 | 0.142 | 23 | 19 | 22 | 0.540 |
| II | 50 | 71 | 59 | 47 | 83 | 50 | 53 | 68 | 59 | ||||
| III | 10 | 31 | 27 | 18 | 22 | 28 | 16 | 28 | 24 | ||||
| Grade | 1 | 26 | 25 | 11 | 0.001 | 17 | 29 | 16 | 0.074 | 17 | 16 | 29 | 0.553 |
| 2 | 31 | 58 | 49 | 43 | 58 | 37 | 47 | 51 | 40 | ||||
| 3 | 24 | 41 | 47 | 22 | 50 | 40 | 28 | 48 | 36 | ||||
| ER status | - | 42 | 70 | 46 | 0.180 | 38 | 71 | 49 | 0.413 | 45 | 63 | 50 | 0.822 |
| + | 39 | 54 | 61 | 44 | 66 | 44 | 47 | 52 | 55 | ||||
| PR status | - | 41 | 71 | 64 | 0.219 | 47 | 76 | 53 | 0.957 | 49 | 68 | 59 | 0.702 |
| + | 40 | 53 | 43 | 35 | 61 | 40 | 43 | 47 | 46 | ||||
Pearson Correlation Test.
Elevated Aurora-B expression is correlated with the poor prognosis in breast cancer patients
To further evaluate whether Aurora-B expression can predict prognosis, we performed a survival analysis of DFS in all patients. Kaplan-Meier survival curves showed that higher expression of Aurora-B significantly correlated with the poor survival in these cases (P = 0.038, Figure 2). A multivariate Cox regression analysis demonstrated that Aurora-B expression is an independent prognostic indicator of breast cancer DFS (HR = 1.39, 95% CI = 1.04-1.86) (Table 4).
Figure 2.
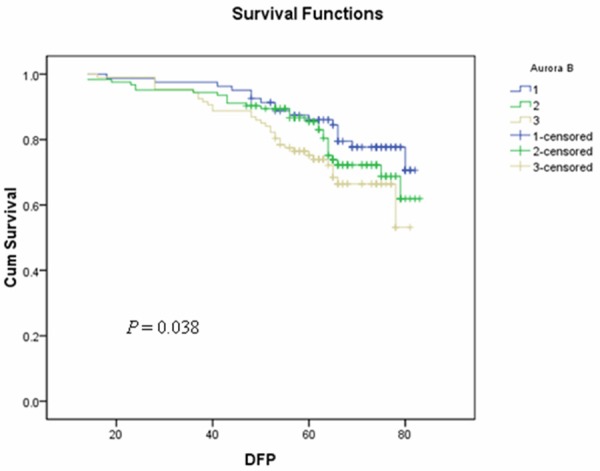
Kaplan-Meier survival curves of the 312 breast cancer patients with different expression levels of Aurora-B. Kaplan-Meier survival curves showed that higher expression of Aurora-B significantly correlated with the poor survival in these cases (P = 0.038).
Table 4.
Cox regression analysis of breast cancer DFS in relation to Aurora-B expression
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| Characteristic | HR (95% CI) | P | HR (95% CI) | P |
| Age | 1.00 (0.98, 1.03) | 0.793 | 0.99 (0.98, 1.02) | 0.790 |
| Stage | 1.99 (1.39, 2.86) | 1.89 × 10-4 | 1.84 (1.27, 2.67) | 0.001 |
| Radiotherapy | 2.05 (1.31, 3.20) | 0.002 | 1.87 (1.19, 2.94) | 0.007 |
| Aurora B | 1.39 (1.04, 1.86) | 0.028 | 1.35 (1.00, 1.83) | 0.050 |
Elevated Aurora-B expression and chemoresistance
To further investigate the clinical relevance of elevated Aurora-B expression with the poor prognosis, we hypothesized that altered expression of Aurora-B might contribute to resistance to chemosensitivity. To test this notion, we analyzed the clinical data and identified 70 patients (70/312, 22.4%) who had received pre-operative neoadjuvant chemotherapy (Table 5). Because these patients have primary tumors that were measurable for their responses to the chemotherapeutic drugs (containing sequential taxane and anthracycline–based regimens), we were able to assess the chemosensitivity and its correlation with expression of Aurora-B and ATM-S1981p. We found that in 39 cases (39/70, 55.7%) who were chemotherapy-resistant (as defined by RCBII/III), 82.1% (32/39) had higher expression of Aurora-B (++~+++). The Pearson correlation test showed that the expression of Aurora-B significantly correlated with the chemoresistance (P = 0.011) (Table 5), indicating a clinical significance of Aurora-B expression. In contrast, ATM-S1981p did not show the correlation with chemoresistant.
Table 5.
Correlations of expression of Aurora B, ATM-S1981p with chemoresistance
| Neoadjuvant chemotherapy (N) | * P | |||
|---|---|---|---|---|
|
|
||||
| chemoresistant | chemosensitive | |||
| Aurora B | -/+ | 7 | 14 | |
| ++ | 14 | 12 | 0.011 | |
| +++ | 18 | 5 | ||
| S1981p-ATM | -/+ | 13 | 9 | |
| ++ | 19 | 10 | 0.137 | |
| +++ | 7 | 12 | ||
Pearson Correlation Test.
Discussion
Defining a biomarker that can predict the clinical response to therapies will be of critical to personalized medicine for breast cancer therapies. In this report, we show for the first time that elevated Aurora-B expression in breast cancer tissues indicates chemoresistance and poor survival, highlighting a predictive value of Aurora-B expression.
There has been debating data regarding the role of the Aurora-kinase A and B in breast cancer prognosis. For instance, an earlier report showed no association of Aurora-A expression with survival in a cohort of 112 patients [10]. However, another report using a larger sample size has demonstrated a strong correlation of high Aurora-A expression, but not Aurora-B, with decreased survival [9]. Our set of data has added more debating points for these studies. Although the differences on the sample size and patient distribution patterns might contribute to variable conclusions, we speculate that the difference of these studies might be primarily attributive to different chemotherapeutic drugs available at the time of treatment. The two studies mentioned above had the enrollment periods of 1986-1993 and 1962-1980, whereas ours are 2005-2006, when in a period that significant changes had been seen regarding available chemotherapeutic drugs. Because Aurora-B’s role in chemoresistance was not previously identified, chemotherapeutic regimens that were available to the patients at the time of treatment should be carefully re-examined.
How elevated Aurora-B expression contributes to chemoresistance remains to be further examined. It is likely that Aurora-B expression promotes the cellular survival in response to chemotherapeutic drugs. It is also possible that Aurora-B overexpression contributes to radioresistance, although the clinical responsiveness of breast cancer radiotherapy is difficult to assess independently in the majority of patients. Further studies in preclinical models are warranted.
Our recently findings have demonstrated Aurora-B’s connection to ATM in mitosis as shown by the dependency of Aurora-B on mitotic ATM Serine 1403 phosphorylation [4]. However, we did not observe the clinical relevance of the two parameters. In contrast, we observed the correlation of Aurora-B expression with p53 expression in these tissues. A recent study has shown negative regulation of Aurora-B in p53, as pharmacological inhibition of Aurora-B in cancer cells with wild-type p53 increased the p53 protein level and expression of p53 target genes to inhibit tumor growth [3]. It is possible that Aurora-B overexpression in breast cancer tissues might selectively up-regulate mutant p53.
In summary, our data shown here provide strong evidence demonstrating that, in breast cancer, elevated Aurora-B expression is associated with chemoresistance and poor survival. Because several Aurora-B Inhibitors have been advanced to clinical trials, identifying Aurora-B as a predicting marker of chemoresistance should provide rationale for designing combination therapy using available chemotherapeutic drugs with these Aurora-B inhibitors.
Acknowledgements
We thank the grant support from National Natural Science Foundation of China (Grant No. 81172531) to Xiaojing Guo, NIH grant R01CA133093 and the Alabama Innovation Fund to Bo Xu, National Natural Science Foundation of China (Grant No. 30930038) to Li Fu, and National Natural Science Foundation of China (Grant No. 81302292) to Xiaolong Qian.
Disclosure of conflict of interest
None.
References
- 1.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–54. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 2.Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H, Sen S, Allis CD, Sassone-Corsi P. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002;22:874–85. doi: 10.1128/MCB.22.3.874-885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gully CP, Velazquez-Torres G, Shin JH, Fuentes-Mattei E, Wang E, Carlock C, Chen J, Rothenberg D, Adams HP, Choi HH, Guma S, Phan L, Chou PC, Su CH, Zhang F, Chen JS, Yang TY, Yeung SC, Lee MH. Aurora B kinase phosphorylates and instigates degradation of p53. Proc Natl Acad Sci U S A. 2012;109:E1513–22. doi: 10.1073/pnas.1110287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang C, Tang X, Guo X, Niikura Y, Kitagawa K, Cui K, Wong ST, Fu L, Xu B. Aurora-B mediated ATM serine 1403 phosphorylation is required for mitotic ATM activation and the spindle checkpoint. Mol Cell. 2011;44:597–608. doi: 10.1016/j.molcel.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C, Wang H, Xu Y, Brinkman KL, Ishiyama H, Wong ST, Xu B. The kinetochore protein Bub1 participates in the DNA damage response. DNA Repair (Amst) 2012;11:185–91. doi: 10.1016/j.dnarep.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C, Hao J, Kong D, Cui X, Zhang W, Wang H, Guo X, Ma S, Liu X, Pu P, Xu B. ATM-mediated Mad1 Serine 214 phosphorylation regulates Mad1 dimerization and the spindle assembly checkpoint. Carcinogenesis. 2014;35:2007–13. doi: 10.1093/carcin/bgu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tatsuka M, Katayama H, Ota T, Tanaka T, Odashima S, Suzuki F, Terada Y. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res. 1998;58:4811–6. [PubMed] [Google Scholar]
- 8.Keen N, Taylor S. Aurora-kinase inhibitors as anticancer agents. Nat Rev Cancer. 2004;4:927–36. doi: 10.1038/nrc1502. [DOI] [PubMed] [Google Scholar]
- 9.Nadler Y, Camp RL, Schwartz C, Rimm DL, Kluger HM, Kluger Y. Expression of Aurora A (but not Aurora B) is predictive of survival in breast cancer. Clin Cancer Res. 2008;14:4455–62. doi: 10.1158/1078-0432.CCR-07-5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Royce ME, Xia W, Sahin AA, Katayama H, Johnston DA, Hortobagyi G, Sen S, Hung MC. STK15/Aurora-A expression in primary breast tumors is correlated with nuclear grade but not with prognosis. Cancer. 2004;100:12–9. doi: 10.1002/cncr.11879. [DOI] [PubMed] [Google Scholar]
- 11.Liedtke C, Hatzis C, Symmans WF, Desmedt C, Haibe-Kains B, Valero V, Kuerer H, Hortobagyi GN, Piccart-Gebhart M, Sotiriou C, Pusztai L. Genomic grade index is associated with response to chemotherapy in patients with breast cancer. J. Clin. Oncol. 2009;27:3185–91. doi: 10.1200/JCO.2008.18.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007;25:4414–22. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 13.Sun M, Guo X, Qian X, Wang H, Yang C, Brinkman KL, Serrano-Gonzalez M, Jope RS, Zhou B, Engler DA, Zhan M, Wong ST, Fu L, Xu B. Activation of the ATM-Snail pathway promotes breast cancer metastasis. J Mol Cell Biol. 2012;4:304–15. doi: 10.1093/jmcb/mjs048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo X, Yang C, Qian X, Lei T, Li Y, Shen H, Fu L, Xu B. Estrogen receptor α regulates ATM Expression through miRNAs in breast cancer. Clin Cancer Res. 2013;19:4994–5002. doi: 10.1158/1078-0432.CCR-12-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]


