Abstract
Objective: To investigate the diagnostic accuracy of the combination of interleukin-33 (IL-33) and adenosine deaminase (ADA) for differentiating TPE from pleural effusions with the other etiologies. Methods: Pleural effusion samples were collected from 32 TPE patients and 55 non-TPE patients. Pleural levels of IL-33 and ADA were measured by ELISA. The corresponding biochemical indexes were also simultaneously determined. Results: The pleural levels of IL-33 and ADA in the TPE group were significantly higher than those in the non-TPE group. With a cut-off value of 68.3 pg/ml, the sensitivity and specificity for IL-33 were 83.9% and 70.9%, respectively. While for ADA, the sensitivity and specificity were 87.5% and 87.3%, respectively at a cut-off value of 10.25 U/L. Combined use of IL-33 and ADA measurements further increased the sensitivity or specificity. Conclusion: Our study suggests that the applications of new biomarker IL-33, along with ADA, may serve as efficient diagnosis strategies in the management of pleural TB. Further studies at a large scale should be performed to validate our findings.
Keywords: Interleukin-33, adenosine deaminase, tuberculosis, pleural effusion, diagnosis
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), is a severe public health problem which remains a major cause of morbidity and mortality throughout the world, especially in Asia and Africa. Tuberculous pleural effusion (TPE) occurs in up to 30% of patients with TB and constitutes the major portion of the extrapulmonary TB morbidity [1]. Distinguishing TPE from non-TPE is crucial in the treatment of patients with pleural effusions (PEs). However, due to the nonspecific clinical presentation and laboratory nature, differential diagnosis of TPE is a diagnostic challenge. Current available diagnostic tools, such as acid fast bacilli test, pleural fluid culture, cytological examination of effusions for inflammatory cells, microbiological and histological examination of biopsied tissue, play a limited role in differentiating TPE from non-TPE [2,3]. Therefore, evaluation of alternative diagnostic strategies has been motivated and mandatory to aid conventional tests.
The IL-1 family member interleukin (IL)-33 is expressed by a variety of stromal cells, including alveolar epithelial cells, endothelial cells, fibroblasts and eosinophilic cells. Recent studies found that IL-33 might amplify both Th1- and Th2-oriented immune responses and was involved in allergic inflammation and asthma [4-6]. Moreover, some investigations showed that IL-33 expression was induced by IFN-γ or tumor necrosis factor (TNF) [7,8], which are increased in the pleural space of patients with TPE [9,10]. Lee et al. [11] also reported that IL-33 was involved in pleural tuberculosis, and showed a significant correlation with the pleural level of conventional TPE marker adenosine deaminase (ADA). So in this study, we have further examined and compared the diagnostic potential of IL-33 and ADA measurements in patients with TPEs. Subsequently, we investigated the accuracy of combining test results to find whether the combination of two values for IL-33 and ADA would improve the diagnostic efficiency.
Methods
Study subjects
In this study, pleural effusion samples were collected from 87 patients who were hospitalized in West China Hospital of Sichuan University from November 2012 to September 2013 for further diagnostic investigation. The study protocol was approved by our Institutional Review Board for Human Studies of West China Hospital of Sichuan University (a 4400-bed comprehensive teaching hospital in Chengdu, Sichuan province, China), and informed written consent was obtained from all subjects.
32 HIV-negative patients were diagnosed with TBE as determined by: (1) positive of acid fast bacilli in pleural fluid, pleural fluid culture shown the growth of Mycobacterium tuberculosis (M. tuberculosis), or demonstration of granulomatous pleurisy on closed pleural biopsy specimen (n = 23); (2) clinical features of tuberculosis infection associated with a sustained positive response to anti-tuberculosis therapy, along with a positive purified protein derivative skin test result and the exclusion of any other potential causes of pleurisy (n = 9).
For the control group, 32 patients with malignant pleural effusions were included. The diagnosis of malignant pleural effusion was made when malignant cells were found on cytological examination and or on closed pleural biopsy, or on lung tissue biopsy. In addition, 12 patients classified with parapneumonic pleural effusions, and 11 patients with pleural effusion caused by heart failure (n = 6), liver cirrhosis (n = 3) or other causes of transudative pleural effusion (n = 2) were also included as control group.
Sample collection and quantification
The pleural effusion samples were collected in 3.2% buffered sodium citrate from each subject within 24 hours after admission, using a standard thoracocentesis technique. Some of the pleural effusion was sent to detect levels of total protein, glucose and lactate dehydrogenase (LDH) in Depart ment of Laboratory Medicine, West China Hospital of Sichuan University, which has met all applicable standards for accreditation and is fully accredited by the College of American Pathologists’ Laboratory Accreditation Program. The other pleural effusion specimens were centrifuged at 2000 g for 10 min at 4°C. The supernatant was stored at -80°C for IL-33 and ADA measurements. The pleural concentrations of IL-33 and ADA were assessed by an enzyme-linked immunosorbent assay following the manufacturer’s instructions (ELISA; Xitang Bio-Technology Co., Ltd., Shanghai, China). All samples were assayed in duplicate. The clinical information of patients was blinded to the operators.
Statistical analysis
Statistical analysis was carried out using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Patient demographics and disease characteristics were summarized using descriptive statistics. Data were expressed as the means ± SD. Differences between independent groups were examined by the Student’s t-test. Receiver operating curve (ROC) analysis was used to evaluate the threshold value of IL-33 and ADA in differentiating TPE from non-TPE. For each ROC, a cutoff point was determined as the value of the parameter that maximized the sum of specificity and sensitivity. A value of P < 0.05 was considered as statistically significant.
Results
Characteristics of the study subjects and pleural effusions
A total of 87 patients were included in the current study. The demographic characteristics of the study subjects are presented in Table 1, and the biochemical characteristics of pleural effusions are shown in Table 2. Patients with TPE showed a marked elevation of pleural protein (P < 0.05), while non-TPE group subjects showed a higher pleural glucose level (P < 0.05).
Table 1.
Clinical and demographic data of the study population
| Age (years) | 55.68 ± 19.24 |
|---|---|
| Sex (male/female) | 59/28 |
| Location | |
| Left | 14 |
| Right | 19 |
| Bilateral | 54 |
| Colour | |
| Yellow | 59 |
| Bloody | 16 |
| Yellow-Bloody | 12 |
| Diagnosis | |
| TPE | 32 |
| non-TPE | 55 |
| MPE | 32 |
| Infectious | 11 |
| Transudative | 12 |
Table 2.
Biochemical characteristics of pleural effusions
| TPE | non-TPE | P-value | |
|---|---|---|---|
| Protein (g/L) | 42.76 ± 10.12 | 33.89 ± 14.14 | 0.003 |
| LDH (U/L) | 384.5 ± 381.79 | 285.16 ± 316.91 | 0.195 |
| Glucose (mmol/L) | 5.39 ± 2.67 | 6.7 ± 2.7 | 0.031 |
| ADA (U/L) | 25.47 ± 19.35 | 7.7 ± 11.41 | < 0.001 |
| IL-33 (pg/ml) | 192 ± 293.75 | 63.45 ± 26.62 | 0.002 |
Diagnostic values of IL-33 and ADA
As shown in Table 2, the concentration of IL-33 in the TPE group (192 ± 293.75 pg/ml) was significantly higher compared to the non-TPE group (63.45 ± 26.62 pg/ml; P < 0.01). Meanwhile, the levels of ADA in TPE (25.47 ± 19.35 U/L) were also significantly higher than those in non-TPE (7.7 ± 11.41 U/L; P < 0.001).
The diagnostic accuracies of pleural IL-33 and ADA were assessed with ROC curve analyses. The area under curve (AUC) of IL-33 to differentiate TPE from all non-TB effusions was 0.823 (95% CI, 0.737-0.909) (Figure 1; Table 3). With a cut-off value of 68.3 pg/ml, the sensitivity and specificity was 83.9% and 70.9%, respectively (Table 3). We also observed that the AUC for ADA to differentiate TPE from non-TPE was 0.930 (95% CI, 0.876-0.985) (Figure 1; Table 3). With a cut-off value of 10.25 U/L, sensitivity was 87.5%, specificity 87.3% (Table 3).
Figure 1.
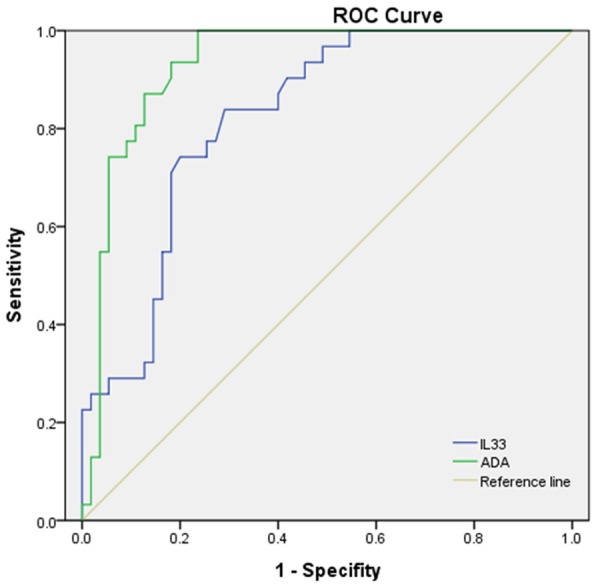
Comparison of diagnostic accuracies of pleural IL-33 and ADA for differential diagnosis of TPE versus non-TPE. Areas under the curves (AUC) were calculated by the trapezoidal rule.
Table 3.
Diagnostic performance of IL-33 and ADA in differentiating tuberculous from non-tuberculous pleural effusions
| Variables | Sensitivity (%) | Specificity (%) | AUC (95% CI) |
|---|---|---|---|
| ADA ≥ 10.25 U/L | 87.5 | 87.3 | 0.930 (0.876-0.985) |
| IL-33 ≥ 68.3 pg/ml | 83.9 | 70.9 | 0.823 (0.737-0.909) |
| ADA or IL-33 | 100 | 69 | - |
| ADA + IL-33 | 75 | 92.7 | - |
Diagnostic value of combined detection of IL-33 and ADA
We also investigate whether the combination of the values for IL-33 and ADA would improve the diagnostic efficiency for differentiating TPE from pleural effusions with the other causes. The combination of these two biomarkers, with a positive result by any one of these biomarkers considered to be indicative of a positive diagnosis of TPE, increased the diagnostic sensitivity for discriminating TPE from all non-TBE (Table 3). However, the combination of IL-33 and ADA, requiring both to be positive for a diagnosis to be made, increased the specificity at the expense of sensitivity (Table 3).
Discussion
Differentiating TPE from non-TPE is still a clinical challenge, since they have similar clinical or laboratory manifestations and sometimes a lack of pathological or etiological evidence [12]. Although histological examination and mycobacterial culture of closed pleural biopsied tissue have been recognized as the gold standard methods of TB diagnosis, and thoracoscopic pleural biopsy can also provide a high sensitivity [2,3], they are invasive and sometimes technically difficult. Therefore, newer rapid tests and biomarkers are necessary and urgent.
The pathophysiology of TPE has been characterized by T-cell-mediated hypersensitivity reaction to mycobacterium or antigens in the pleural space. This process involves the accumulation of protein-enriched fluid and the migration of immune cells that are generally T lymphocytes and macrophages [13-15]. IL-33 has recently gained much attention due to its role in immune responses. It has been shown that IL-33 is a highly inflammatory cytokine constitutively expressed in mucosal or barrier cell types, acting as regulators of innate and acquired immune responses by amplifying both Th1 and Th2 responses with or without TCR activation [16,17]. IL-33 stimulation of Th1 cultures resulted in increased IFN-γ [18]. In addition, it also directly induced IFN-γ production from both iNKT and human NK cells via cooperation with IL-12 [19]. On the other hand, IL-33 expression could be up-regulated by IFN-γ and TNF-α [7,8]. As TPE is a Th1-dominant environment, and Th1 cytokines such as IFN-γ and TNF-α predominate at pleural effusions in patients with TPE [20,21], questions regarding the IL-33 response in tuberculous pleural diseases arise when there have been accumulated evidence and conflicting hypotheses for the involvement of IL-33 in inflammatory and immune pathogenesis.
In the present study, our data have shown that pleural IL-33 levels in TPE are much higher than those with other etiologies of pleural effusions, indicating the involvement of IL-33 in the pathogenesis of TPE. These results agree with those of recent studies by Lee et al [11] and Xuan et al [22], which found that pleural and serum IL-33 levels were higher in patients with TPE compared with those patients with other types of pleural effusions. Although the mechanism of IL-33 contribution to TPE pathogenesis is still not clear, it is plausible to hypothesize that IL-33 may induce or be enhanced by specific cytokines caused by tuberculosis rather than by other etiologies, such as IFN-γ, and the process forms a positive feedback loop in tuberculous pleurisy. We also investigated the diagnostic value of IL-33 in distinguishing tuberculous from pleural effusions of other etiologies. Nevertheless, our data showed that using a threshold value of 68.3 pg/ml, IL-33 had a sensitivity of 83.9% and a specificity of 70.9%, respectively, suggesting that pleural IL-33 was not an efficient diagnostic marker for detection of TPE.
ADA is one of the most widely studied and recommended biomarkers and has been found to have a good performance at diagnosing TPE. A meta-analysis of 63 studies, which assessed the value of pleural fluid ADA activity in differentiation between TPE and non-TPE, demonstrated a high sensitivity and specificity of these measurements (92 and 90%, respectively) [23]. In the present study, we found good diagnostic sensitivity and specificity of ADA for detecting TPE (87.5% and 87.3%), respectively, which were similar to those of previous studies [24,25].
The combined diagnostic value of the IL-33 and ADA in TPE and non-TPE was further analyzed. The results showed that the combined detection of these two indices, which required at least one of these measurements to be positive, resulted in an optimal sensitivity of 100%, whereas a specificity of 89% was found in a combination that required both of these two measurements to be positive. Both sensitivity and specificity were higher compared to the separate test for TPE and non-TPE, indicating that the combined use of IL-33 and ADA had a better diagnostic value than the use of a single index. This may provide a new approach in the differential diagnosis of TPE.
Our study has several limitations, first of all, we enrolled only 32 patients with TPE; the limited patients number may affect the application of our findings. Secondly, our study is an observational study; we didn’t analyze serial IL-33 levels or measure Th2 and Th1 cytokines such as IFN-γ in PEs. Thirdly, we did not do a further work on the detail mechanism how IL-33 affects the pleural immune reaction and inflammatory response. Further studies at a large scale and aiming to investigate the detailed mechanism should be carried out to confirm our findings.
In summary, our results suggest that the levels of IL-33 and ADA are higher in TPE than those in non-TPE. Although IL-33 had a lower diagnostic accuracy compared with ADA, combinations of the two methods yielded satisfactory sensitivity or specificity. These findings suggest that the applications of new biomarker IL-33, along with ADA, may serve as more efficient diagnosis strategies in the management of pleural TB.
Acknowledgements
This work was supported by grants 81230001 and 81300032 from the National Natural Science Foundation of China.
Disclosure of conflict of interest
None.
References
- 1.Hasaneen NA, Zaki ME, Shalaby HM, El-Morsi AS. Polymerase chain reaction of pleural biopsy is a rapid and sensitive method for the diagnosis of tuberculous pleural effusion. Chest. 2003;124:2105–2111. doi: 10.1378/chest.124.6.2105. [DOI] [PubMed] [Google Scholar]
- 2.McGrath EE, Anderson PB. Diagnostic tests for tuberculous pleural effusion. Eur J Clin Microbiol Infect Dis. 2010;29:1187–1193. doi: 10.1007/s10096-010-0986-z. [DOI] [PubMed] [Google Scholar]
- 3.Light RW. Update on tuberculous pleural effusion. Respirology. 2010;15:451–458. doi: 10.1111/j.1440-1843.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 4.Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, Lukic ML. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52:89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 5.Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy. 2010;40:200–208. doi: 10.1111/j.1365-2222.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- 6.Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemière C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 7.Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun. 2009;384:105–109. doi: 10.1016/j.bbrc.2009.04.081. [DOI] [PubMed] [Google Scholar]
- 8.Masamune A, Watanabe T, Kikuta K, Satoh K, Kanno A, Shimosegawa T. Nuclear expression of interleukin-33 in pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G821–32. doi: 10.1152/ajpgi.00178.2010. [DOI] [PubMed] [Google Scholar]
- 9.Qama D, Choi WI, Kwon KY. Immune responses in the lungs of patients with tuberculous pleural effusion without pulmonary tuberculosis. BMC Immunol. 2012;13:45. doi: 10.1186/1471-2172-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong CF, Yew WW, Leung SK, Chan CY, Hui M, Au-Yeang C, Cheng AF. Assay of pleural fluid interleukin-6, tumour necrosis factor-alpha and interferon-gamma in the diagnosis and outcome correlation of tuberculous effusion. Respir Med. 2003;97:1289–1295. doi: 10.1016/j.rmed.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Lee KS, Kim HR, Kwak S, Choi KH, Cho JH, Lee YJ, Lee MK, Lee JH, Park SD, Park DS. Association between elevated pleural interleukin-33 levels and tuberculous pleurisy. Ann Lab Med. 2013;33:45–51. doi: 10.3343/alm.2013.33.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu YB, Ye ZJ, Qin SM, Wu C, Chen YQ, Shi HZ. Combined detections of interleukin 27, interferon-γ, and adenosine deaminase in pleural effusion for diagnosis of tuberculous pleurisy. Chin Med J (Engl) 2013;126:3215–3221. [PubMed] [Google Scholar]
- 13.Barnes PF, Mistry SD, Cooper CL, Pirmez C, Rea TH, Modlin RL. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989;142:1114–1119. [PubMed] [Google Scholar]
- 14.Sharma SK, Mitra DK, Balamurugan A, Pandey RM, Mehra NK. Cytokine polarization in miliary and pleural tuberculosis. J Clin Immunol. 2002;22:345–352. doi: 10.1023/a:1020604331886. [DOI] [PubMed] [Google Scholar]
- 15.Antony VB, Repine JE, Harada RN, Good JT Jr, Sahn SA. Inflammatory responses in experimental tuberculosis pleurisy. Acta Cytol. 1983;27:355–361. [PubMed] [Google Scholar]
- 16.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 17.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blom L, Poulsen LK. IL-1 family members IL-18 and IL-33 upregulate the inflammatory potential of differentiated human Th1 and Th2 cultures. J Immunol. 2012;189:4331–7. doi: 10.4049/jimmunol.1103685. [DOI] [PubMed] [Google Scholar]
- 19.Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, Herbelin A. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–55. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 20.Hooper CE, Lee YC, Maskell NA. Interferon-gamma release assays for the diagnosis of TB pleural effusions: hype or real hope? Curr Opin Pulm Med. 2009;15:358–365. doi: 10.1097/MCP.0b013e32832bcc4e. [DOI] [PubMed] [Google Scholar]
- 21.Seiscento M, Vargas FS, Acencio MM, Teixeira LR, Capelozzi VL, Sales RK, Antonangelo L. Pleural fluid cytokines correlate with tissue inflammatory expression in tuberculosis. Int J Tuberc Lung Dis. 2010;14:1153–1158. [PubMed] [Google Scholar]
- 22.Xuan WX, Zhang JC, Zhou Q, Yang WB, Ma LJ. IL-33 levels differentiate tuberculous pleurisy from malignant pleural effusions. Oncol Lett. 2014;8:449–453. doi: 10.3892/ol.2014.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang QL, Shi HZ, Wang K, Qin SM, Qin XJ. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med. 2008;102:744–54. doi: 10.1016/j.rmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Teo SK, Chio LF. Adenosine deaminase in pleural fluid-an enzymatic test for tuberculous pleural effusion. Singapore Med J. 1987;28:220–4. [PubMed] [Google Scholar]
- 25.Burgess LJ, Maritz FJ, Le Roux I, Taljaard JJ. Combined use of pleural adenosine deaminase with lymphocyte/neutrophil ratio. Increased specificity for the diagnosis of tuberculous pleuritis. Chest. 1996;109:414–419. doi: 10.1378/chest.109.2.414. [DOI] [PubMed] [Google Scholar]


