Abstract
Goals: To evaluate the therapeutic efficacy of rat bone marrow mesenchymal stem cells (BMSCs) induced into hepatocyte-like cells and of un-induced BMSCs in acute liver failure rats. Methods: BMSCs in highly homogenous passage 3 were cultured using the whole bone marrow adherent culture method. Hepatic-related characters were confirmed with morphology, RT-PCR analysis, glycogen staining and albumin (ALB) immunofluorescence assay. Carbon tetrachloride (CCl4) was injected intraperitoneally to establish an acute rat liver failure model. Hepatocyte-like cells or un-induced BMSCs were respectively injected into the models to examine rats’ appearance, liver function assay and liver tissue pathology. Results: Hepatocyte-like morphology, higher expression of cytokeratin 18 (CK18) mRNA and ALB protein, and glycogen accumulation were confirmed in the induced BMSCs. The transplanted DAPI-labeled BMSCs were localized in the liver tissue 3-14 days after transplantation. The levels of liver function indicators (AST, ALT, ALP, and TBIL) from transplanted rats were significant decreased and pathology was improved, indicating the recovery of liver function. However, the differences were statistically insignificant. Conclusion: Both hepatocyte-like cells and un-induced BMSCs had a similarly positively therapeutic efficacy on liver regeneration in rat liver failure model.
Keywords: Bone marrow mesenchymal stem cells, hepatocyte-like cells, hepatocyte growth factors, acute liver failure, rats
Introduction
Bone marrow mesenchymal stem cells (BMSCs) are self-replicating and pluripotent stem cells; under different inducing conditions they can differentiate into bone cells [1], adipocytes [2], nerve cells [2], cardiomyocytes [2], and chondrocytes [3] among others. BMSCs are also reported to differentiate into hepatocytes [4-6]. To induce BMSCs to differentiate into hepatocyte-like cells in vitro, growth factors like hepatocyte growth factor (HGF) and fibroblast growth factor 4 (FGF-4) are crucial supplements during the differentiation process [7-9]. HGF is mainly produced in liver Kupffer cells and sinusoidal endothelial cells. In hepatocytes, it induces mitosis, DNA synthesis, cell growth, migration, and morphological changes playing an important role in liver development and regeneration. Therefore, HGF is widely used to induce liver-related stem cell differentiation in vitro [10-12]. FGF-4 is multi-functional cell growth factor and secreted by endothelial cells, smooth muscle cells, and macrophages. It induces endothelial cell migration and smooth muscle cell proliferation [13,14]. Other factors, such as insulin-transferrin-selenium (ITS), and nicotinamide were also reported to play roles in hepatocyte development and regeneration [6]. However, it is still unclear whether BMSCs induced with these growth factors, have a greater effect on hepatocyte regeneration after liver failure than un-induced BMSCs. The most common methods for BMSCs isolation are density gradient centrifugation, whole bone marrow adherent culture, flow cytometry, and immunomagnetic isolation. Cells obtained via density gradient centrifugation are relatively homogeneous, but have poor pluripotency and proliferation ability [15]. Whole bone marrow adherent cultures lead to cells that proliferate quickly, allowing the acquisition of a large amount of BMSCs in a short time period [16]. For detection of hepatocyte differentiation, Albumin (ALB), α-fetoprotein (AFP), cytokeratin 18 (CK18), and glycogen are unique markers indicating the presence and metabolic activity of liver cells. AFB and ALB are both cytoplasmic proteins; AFP is secreted by liver precursor cells, and its secretion level decreases as the cells mature; ALB is produced by hepatocytes, and its expression increases as cells mature. CK18 is a keratin relatively unique to hepatocytes, which is not expressed in liver precursor cells but is expressed in hepatocytes in the process of maturation or that are already mature [17]. Glycogen synthesis is a function unique to mature hepatocytes, because only hepatocytes can produce and store glycogen. Ki-67 is a nuclear protein closely related to cell proliferation. Since it is only expressed in proliferating cells and not expressed in quiescent cells, its abundance is reflecting the cell’s proliferative activity and regarded as a reliable indicator of cell proliferation. In our study we used these markers to isolate BMSCs and monitor their differentiation into hepatocytes.
Despite a large number of studies on the isolation, purification, and differentiation of BMSCs in recent years [18-20], much research is still needed to establish a stable culture method for extensive clinical applications. Particularly improving the efficiency of induced differentiation is crucial for determining whether differentiated hepatocyte-like cells have the same ability to grow in severely damaged livers and to help the liver regenerate and recover as compared to un-induced BMSCs. In this study, using a rat liver failure model we tested our hypothesis that BMSCs induced to differentiate into hepatocyte-like cells in vitro, might produce a better therapeutic outcome than un-induced.
Materials and methods
Isolation, purification, and culture of bone marrow stem cells
10% chloral hydrate was injected into the peritoneal cavity of Sprague-Dawley (SD) rats with body weights between 80-100 g at a dosage of 0.3 ml/100 g. While the animal was still breathing, an incision was made in the skin to expose the femur and tibia keeping the bone marrow cavity intact. PBS was used to wash away blood and hair, and muscles were detached. The bones were then removed, and the bone marrow cavity was exposed. To collect the bone marrow, the bone marrow cavity was washed twice using a full syringe containing DMEM/F12 culture medium with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin. The eluted medium was filtered through a 200-mesh stainless steel filter. The filtrate was mixed well to create a single cell suspension, and then plated onto a 25 cm2 flask for cell culture and placed in a constant temperature incubator at 37°C, 5% CO2 with saturated humidity. After 3 days, the growth medium was completely changed, and cells were examined under an inverted microscope to observe the growth status. Using the method of selecting for adherent cells repeatedly, the adherent BMSCs were finally obtained. Thereafter, the growth medium was changed every 2 days. 7-10 days later the cells converged with each other, and after 10-14 days, when the first generation cells reached 90% confluence, they were passaged for the first time (P1). They were then passaged once every 3 days and the passage 3 (P3) cells were selected for further experiments.
Flow cytometry analysis using BMSCs markers
BMSCs are CD29 and CD90 positive, but negative for the CD34 and CD45 hematopoietic lineage surface markers [21], which we used for their detection. Flow cytometry analysis was performed with a FACSCalibur flow cytometer (Becton Dickinson, CA, USA). A total of 106/100 μl P3 BMSCs were labeled with the directly conjugated antibodies CD34-PE, CD45-PerCP, CD29-FITC and CD90-PE (Biolegend, US).
Inducing the differentiation of BMSCs into hepatic cells
Passage 3 BMSCs reaching 90% confluence were used for differentiation assays. Cells were cultured in differentiation media of DMEM/F12 supplemented with 20 ng/ml HGF, 10 ng/ml FGF-4 (Peprotech, US), 10-7 μmol/L dexamethasone (Dex), 1× insulin-transferrin-selenium (ITS), 0.73 g/Lglutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen); the media were refreshed twice weekly. Hepatic differentiation was assessed at 7, 14 and 21 days after induction with Periodic acid-Schiff (PAS) glycogen immunofluorescence staining and albumin immunofluorescence.
Immunofluorescence and immunohistochemical analysis
Immunostaining was performed by the Avidin Biotin Complex (ABC) method as previously described [22]. A PAS staining kit for glycogen was purchased from Sigma-Aldrich and used according to the manufacturer’s instructions. Stained cells were observed under an inverted phase contrast microscope. Primary and secondary antibodies used for immunofluorescence were mouse polyclonal anti-ALB (1:100, R & D) and rabbit polyclonal anti-mouse IgG FITC (1:100, Sigma). For immunohistochemical staining, the sections were incubated with rabbit polyclonal anti-mouse Ki-67 (1:200, R & D) and goat polyclonal anti-rabbit IgG (1:100, Sigma). The sections were mounted and examined with a fluorescence microscope (Nikon, Japan), and the images were processed with Adobe Photoshop software.
q-RT-PCR
Following the manufacturer’s protocol, RNA was extracted from cells with Trizol reagent (Invitrogen) and reverse-transcribed using a reverse transcription kit (Invitrogen, GBR). Normal liver tissue and non-induced BMSCs were used as a positive and a negative control, respectively. Quantitative Real-time PCR q-RT-PCR analyses were performed employing an ABI Prism 7000 Sequence Detection System with the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and AFP, ALB, CK-18 and GAPDH specific primer. The relative expression of each gene was normalized with GAPDH expression.
Establishing of a rat liver failure model
Seventy SD rats (64 rats 200-250 g, 6 rats 60-80 g) were purchased from SHANGHAI SLAC LABORATORY ANIMAL CO. LTD (Chinese Academy of Sciences, Shanghai, China). Animal experiments were performed in compliance with federal Chinese laws and the animal guidelines of the Fuzhou PLA General Hospital. To induce liver failure, 4.0 ml/kg carbon tetrachloride (CCl4) (Tianjin Beifang Asclepius Reagent Factory, China) mixed with olive oil (1:1 ratio) was injected intraperitoneally into rats which had been fasted without water for 12 h. After 24 h, we confirmed that the model had been successfully established by observing the animal behavior and by performing cytology of the liver cells.
Hematoxylin and eosin (HE) staining
Liver tissue was harvested after perfusion with 4% paraformaldehyde solution and preserved in formalin buffer solution. For HE staining liver tissue was paraffin embedded, sectioned into 4-mm thick slices, placed on the slides with neutral resin and stained. After sealing the slides, the stained tissue slices were microscopically examined at 200× magnification. Subsequently, color images of five randomly chosen microscopic fields on each slice were captured and analyzed by a medical imaging software program (Image-Pro Plus, Media Cybernetics, USA) to semi-quantitatively determine the areal density (AD).
Labeling BMSCs with DAPI
In order to temporarily trace the BMSCs we used the fluorescent marker 4’,6-diamidino-2-phenylindole (DAPI) (Biolegend, US) to label them. Upon the P3 BMSCs reaching 80-90% confluence or 14 days after hepatocyte induction, DAPI solution was added to the growth medium to a final concentration of 1 µg/ml. After overnight incubation, cells were washed with PBS at least 6 times to remove any unbound DAPI, and observed under a fluorescence microscope to confirm the labeling.
Transplantation of the BMSCs
24 hour after being subjected to the liver failure procedure, the rats were randomly divided into three groups: BMSC transplantation group (17 rats), induced BMSC transplantation group (17 rats), and a liver failure control group (17 rats). 1 ml of cell suspension containing 2 × 106 DAPI-labeled BMSCs or DAPI-labeled 14d-post induction BMSCs was slowly injected into the rats via a tail vein. An equal amount of saline solution was injected into the rats in a control group. Three rats from each group were sacrificed at day 3, day 7 and day 14 after BMSC transplantations to track their location and differentiation.
Post-transplantation liver function assessment
At 3 d, 7 d and 14 d after the transplantation, the rats in each group were anesthetized with a 0.3 ml/100 g intraperitoneal injection of 10% chloral hydrate and blood samples were taken from the posterior vena cava. After centrifugation, the serum was collected for assessment of albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), and alkaline phosphatase (ALP) levels using a fully automated biochemical analyzer.
Observing the growth of transplanted cells in the liver
At 3 d, 7 d and 14 d after transplantation, liver tissue samples were collected and frozen slides were prepared. DAPI-labeled BMSCs in the liver tissue were localized under a fluorescence microscope at 100× magnification.
Statistical analysis
The SPSS 11.5 statistical software was used for all analyses. All data are shown as X̅ ± sd. The T-test was used to compare two sets of mean values and the ANOVA method was used to compare multiple mean values between different time points. P < 0.05 was considered statistically significant.
Results
Isolation, purification, cell culturing, and identification of BMSCs
BMSC of the P3 generation were assessed by flow cytometry to confirm their BMSC status. As shown in Figure 1, 0.48% of the cells were CD34 positive and 0.69% of were CD45 positive, while 98.7% of the cells were CD29 positive and 99.2% were CD90 positive; these confirmed that the cells were BMSCs of high purity.
Figure 1.
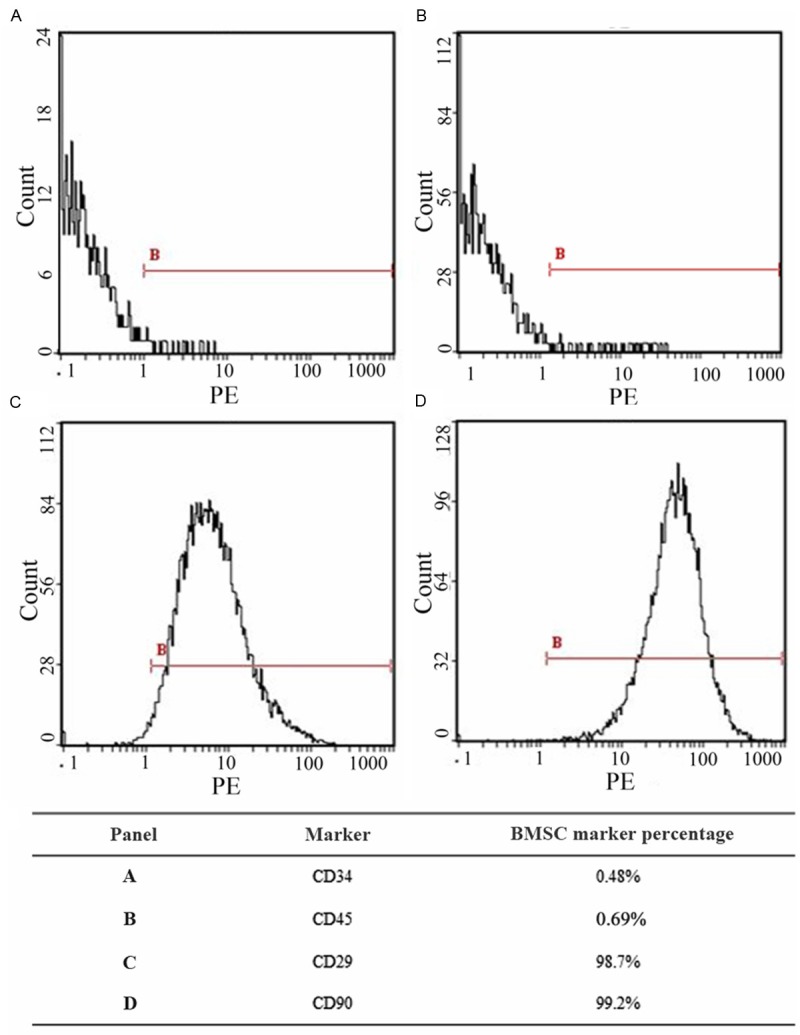
Flow cytometry assessment of the ratio of cells positive for BMSC markers. BMSCs are CD29+, CD90+ and CD34-, CD45-.
Induction of differentiation into hepatocyte-like cells
P3 generation BMSCs cultured in differentiation medium for 3-4 days were subjected to optical microscopic observation. Clusters of cells were observed, and a change in morphology to a spindle shape with a visible nucleus was beginning to take place (Figure 2A). After 7 days, this morphology change became more obvious, and cells showed a swirling pattern of growth (Figure 2B). After multiple passages, the number of cells exhibiting differentiated morphology increased. 14 days after induction, some cells were triangular or circular in shape (Figure 2C). After 21 days, most cells became round, and started to form clusters, exhibiting hepatocyte-like morphologies (Figure 2D).
Figure 2.
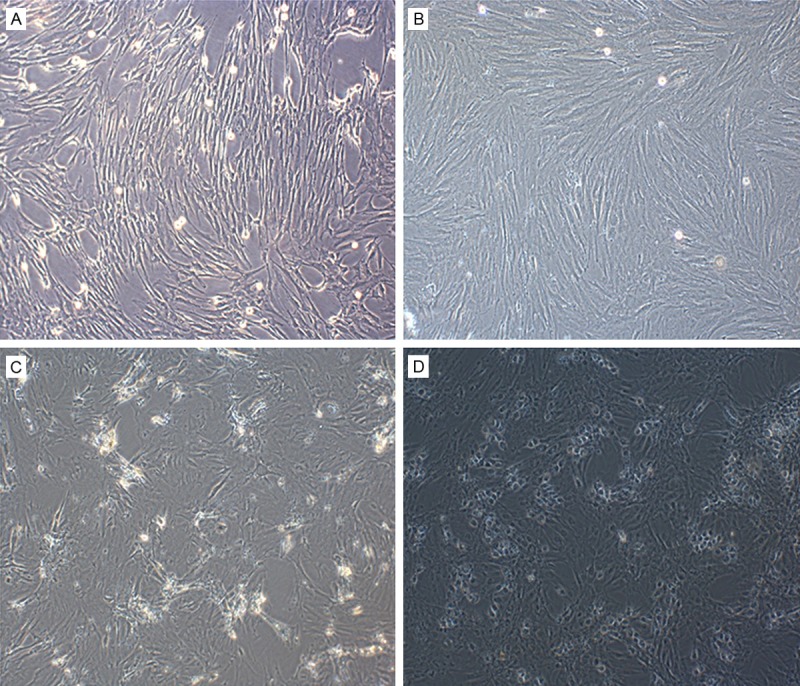
Morphology changes of BMSC during passaging and induction of differentiation (100× magnification). A: First generation BMSCs. B: BMSCs at P3. C: 14 d after induction. D: 21 d after induction.
In order to verify hepatic differentiation on the molecular base, we used q-RT- PCR to determine AFP, ALB, and CK18 mRNA levels (Figure 3A-C). We found that in the un-induced BMSC population, AFP mRNA was highly expressed while ALB and CK18 mRNA levels were low. During culturing in differentiation medium containing 20 ng/ml HGF, 10 ng/ml FGF-4, 10-7 µmol/L Dex and 1xITS, the BMSCs began to show characteristics of hepatocytes with elevated levels of ALB and CK18 mRNA. The transcription levels of AFP mRNA reached a maximum at 14 days and then decreased at day 21. This decrease in AFP mRNA levels suggested maturation of the induced BMSCs, since AFP is not expressed in mature hepatocytes. To further determine the expression of hepatic markers, PAS staining and AIb immunofluorescence assays were performed. The result showed that ALB and CK18 expression and glycogen accumulation levels in BMSCs increased with time after induction of differentiation (Figures 3D-F, 4). These results demonstrated that the BMSCs cultured in the differentiation medium underwent differentiation and became mature hepatocytes, suggesting that the induction method, which we employed, was successful.
Figure 3.
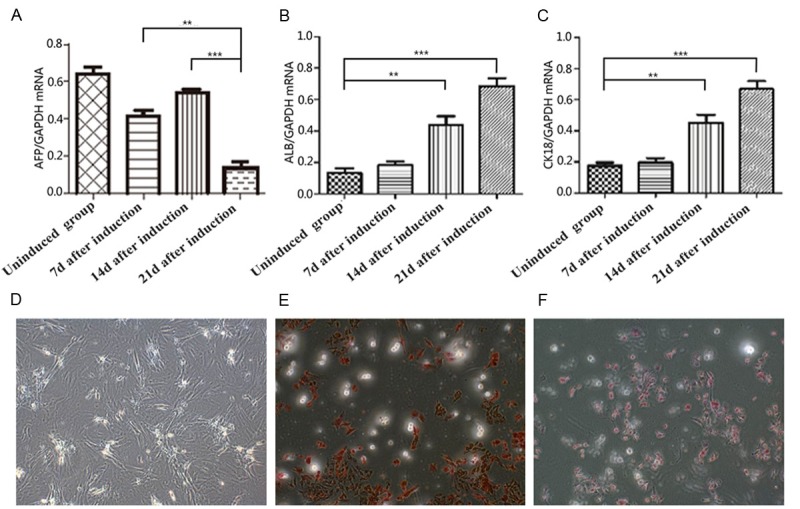
Expression levels of mRNA encoding hepatocyte-like cell markers and PAS glycogen staining (100× magnification) during induction of hepatic differentiation. A: AFP mRNA levels. B: ALB mRNA levels. C: CK18 mRNA levels. D: Glycogen staining 7 days after induction (no positive cells). E: Glycogen staining 14days after induction. F: Glycogen staining 21 days after induction; **P < 0.01, ***P < 0.001.
Figure 4.
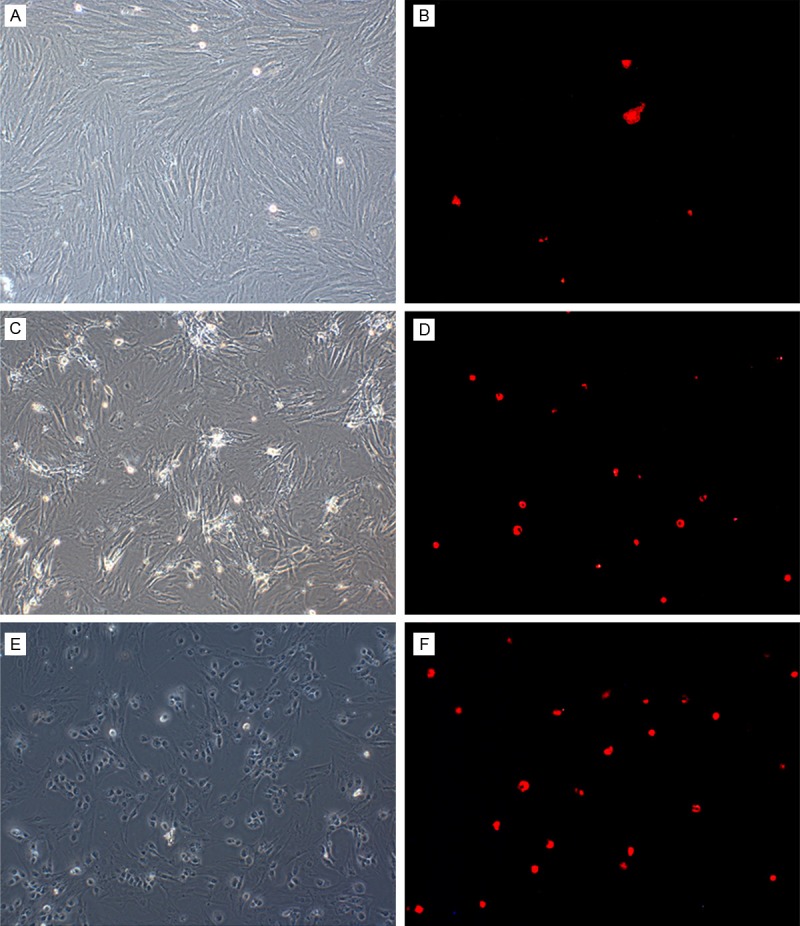
ALB immunofluorescence assay. A: Uninduced BMSCs before staining. B: Uninduced BMSCs after staining. C: BMSCs 14 d after induction, before staining. D: BMSCs 14 d after induction, after staining. E: BMSCs 21 d after induction, before staining. F: BMSCs 21 d after induction, after staining.
Establishment of a liver failure rat model
To create an acute liver failure animal model, CCl4 was injected into the rats. 4 hours after injection the animals became less active exhibiting arched backs, reduced appetite, lack of energy, no resistance to handling, less aggressive and secreted yellow urine. After 8 hours, 60% of the treated animals died. Necropsy showed that these rats had ascites as well as distended and bloody intestines with some level of adhesion.
As has been described previously [23], we observed necrosis of centrilobular hepatocytes, characterized by cell shrinkage and lost nuclei in the CCl4-treated rats, demonstrating that the model had been reproduced successfully in our experiment (Figure 5).
Figure 5.
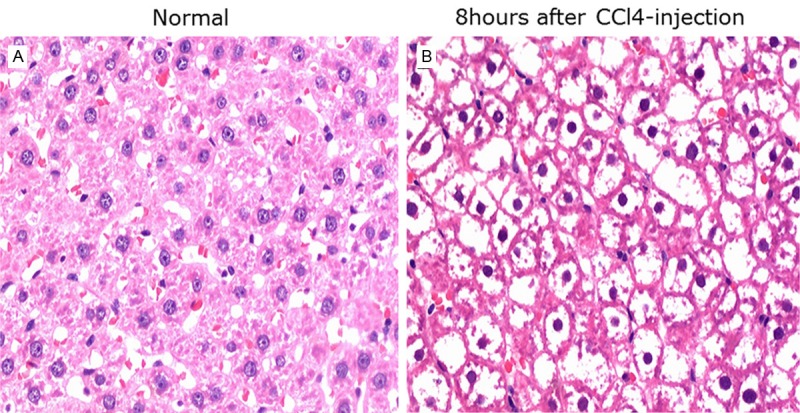
Comparison of liver histology between the control group and the liver failure model group 8 h after CCl4 injection.
Our liver function assays showed, that in the serum from liver failure rats, AST, ALT, ALP, and TBIL levels were significantly elevated compared to the control healthy rats group, while ALB levels were not significantly different from the control group (Table 1). The result suggested that after liver damage, AST, ALT, ALP, and TBIL levels all increased within a short period of time, indicating that liver function has begun to fail.
Table 1.
Liver function analysis in healthy control and liver failure rats
| AST (U/L) * | ALT (U/L) * | ALB (g/L) *** | TBIL (mmol/L) ** | ALP (U/L) * | |
|---|---|---|---|---|---|
| Healthy control rats | 141.90 ± 47.99 | 68.20 ± 17.40 | 39.30 ± 2.05 | 0.56 ± 0.42 | 192.80 ± 67.89 |
| Liver failure ratsa | 423.20 ± 152.87 | 298.80 ±184.39 | 28.54 ± 3.37 | 5.64 ± 2.23 | 306.80 ± 92.43 |
4 days after CCl4 treatment the serum was prepared and subjected to the analysis.
3 rats from each group were analyzed;
P < 0.02;
P = 0.01 (t = 5.060);
P = 0.000 (t = 7.757).
BMCS transplantation enhanced the recovery of CCl4 induced liver damages
In the serum from liver failure rats, 3 days after transplantation of BMSCs or induced BMSCs, the levels of AST, ALT, TBIL and ALP all decreased significantly (P < 0.05). This pattern was maintained to lesser extent at day 7. At day 14 no significant difference between all groups could be observed (P > 0.05). ALB levels were higher at day 3 and day 7 after transplantation in the treated groups as compared to the saline control group (P < 0.05). Between the BMSC and the induced BMSC transplantation groups, at all three time points there were no statistically significant differences in the levels of AST, ALT, TBIL and ALP. However, the levels of ALB were slightly higher in the induced BMSC group at day 3 and day 7 (P < 0.05). Thus BMSC and induced BMSC transplantation could significantly decrease AST, ALT, ALP, and TBIL levels while elevated ALB level in the serum, indicating their effectiveness for liver function recovery (Figure 6).
Figure 6.
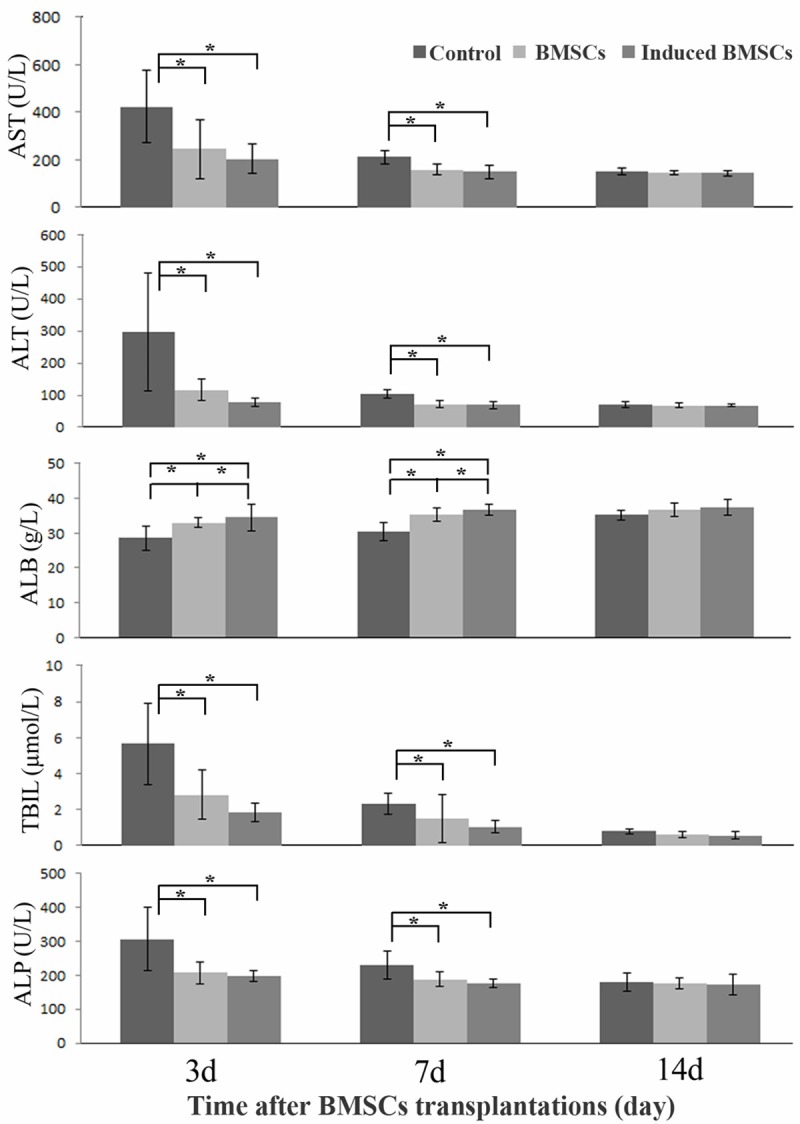
Liver function analysis in CCl4 treated rats after BMSC and induced BMSC transplantations. The control rats received saline solution but not any BMSCs. *P < 0.05. At the 14 d point the differences between treatment groups were statistically insignificant (P > 0.05).
We further examined the liver tissues of induced BMSC transplanted liver failure rats. HE staining showed that 3 days after transplantation, proliferating cells, such as double-nuclei cells, appeared around the point necroses. A few cells underwent vacuolar degeneration, and there was some inflammatory cell infiltration in the periportal region in the un-transplanted control group. In the induced BMSC transplanted group after 3 days, the hepatocytes were swelling, the cytoplasm was becoming diluted, cells exhibited a balloon-like morphology, while the liver sinuses expanded and became filled with blood. Dispersed inflammatory cell infiltration and point necroses were still visible (Figure 7B). Seven days after transplantation, there was a liver histology improvement (Figure 7C) and 21 days after transplantation, the lobular structure had mostly returned to normal. At the 3 day time point, no significant difference was observed in the pathology of the BMSC and the induced BMSC transplant groups, however, both groups showed significantly better liver tissue pathology than the liver failure control group (Figure 7A).
Figure 7.
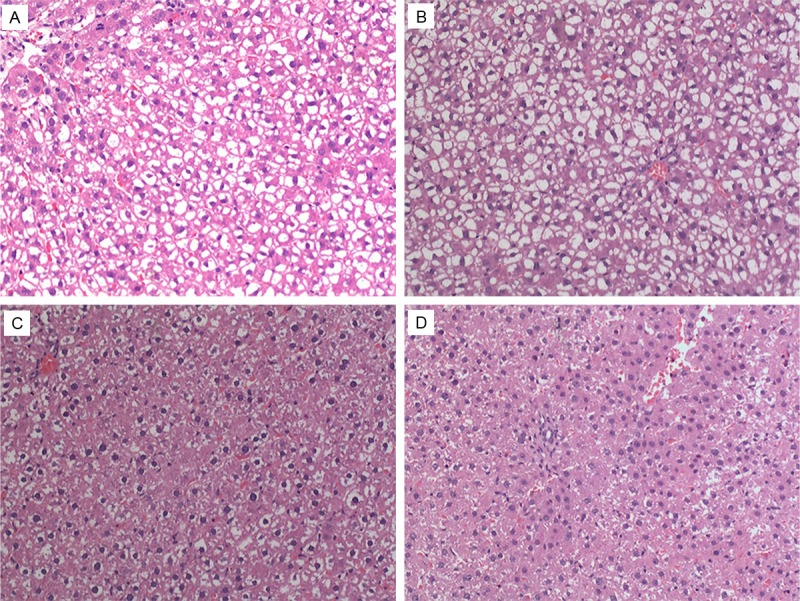
Liver tissue pathology HE staining (100× magnification) at different days after BMSC transplantation. A: Liver failure model control group at the 3 day time point. B: Liver tissue of induced BMSC transplanted rats 3 days after transplantation. C: Liver tissue of induced BMSC transplanted rats 7 days after transplantation. D: Liver tissue of induced BMSC transplanted rats 21 days after transplantation.
Ki-67 expression in liver tissue
Ki-67 is a nuclear protein closely related to cell proliferation. Since it is only expressed in proliferating cells, we predicted that Ki-67 levels would be low in damaged liver tissue. To evaluate recovery of liver cells, the Ki-67 expression levels in liver tissue from liver failure control rats, BMSC and induced BMSC transplanted rats were measured by immunohistochemistry. We found that at the 3day time point of transplantation, Ki-67 expression levels were higher in both the BMSC (162.20 ± 20.49 cells/400× field of vision) and the induced BMSC transplant group (194.82 ± 17.59 cells/400× field of vision) compared to the control group (129.4 ± 11.05 cells/400× field of vision), and the difference was statistically significant (F = 18.478, P = 0.000). At 7 days after transplantation, the Ki-67 expression levels in the BMSC transplant group (31.60 ± 6.20 cells/400× field of vision) and the induced BMSC transplant group (36.80 ± 6.79 cells/400× field of vision) still appeared to be higher than in the control group (18.80 ±5.54 cells/400× field of vision), but the difference was not statistically significant (F = 10.473, P = 0.002). Fourteen days after transplantation, Ki-67 expression levels were similar in all three groups (F = 1.174, P = 0.342) (Figure 8 and data not shown).
Figure 8.
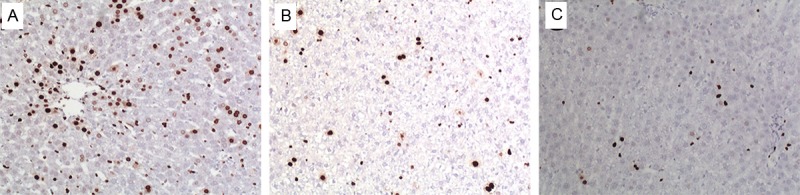
Ki-67 expression in liver tissue after transplantation with induced BMSCs (100×). A: 3 d after transplantation. B: 7 d after transplantation. C: 14 d after transplantation.
Localization of DAPI-labeled BMSCs in the liver
To localize the transplanted BMSCs in the rat liver, we labeled BMSCs with DAPI prior to the transplantation. We observed DAPI-labeled BMSCs in the transplanted liver tissue under a fluorescence microscope, and found that at 3 days after transplantation, BMSCs entered the interior of the liver from the blood vessels and were dispersed among the hepatocytes (Figure 9A). This shows that a portion of the BMSCs, after injection through the tail vein traveled through the systemic circulation and was able to localize in the liver. The number of DAPI-labeled fluorescent cells gradually decreased from the 3 day to 14 day time point (Figure 9B), but this may have been due to the photobleaching of the fluorescence of DAPI.
Figure 9.
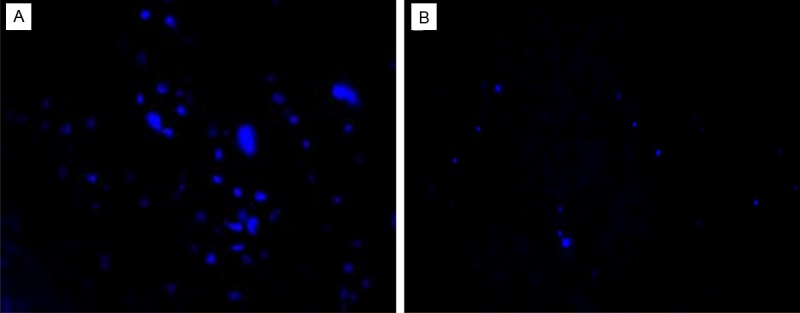
BMSC localization in the liver (100×). A: 3 d after transplantation. B: 14 d after transplantation.
Discussion
Though there have been a number of reports of in vitro culture of BMSCs with α-MEM, DMEM, and SD growth media, we found through pre-experimentation that DMEM/F12 (1:1) was the best base culture medium for our study. This medium enabled the first generation cells to be kept undisturbed for 3 days and produced the most BMSCs prior to the first medium change, and also allowed a faster proliferation rate later on. After multiple passages, over 95% of the cells expressed CD29 and CD90 while less than 2% of the cells expressed CD34 and CD45. With respect to differentiation into specific cell types, components of the growth medium are still being under investigation. One of the reports claimed that factors such as HGF, FGF-4, and TGF-a could induce BMSCs to differentiate into hepatocyte-like cells [10,12]. Based on their findings, we supplemented the growth medium with factors such as hepatocyte growth factor (HGF), fibroblast growth factor (FGF-4), insulin-transferrin-selenium (ITS) and dexamethasone (Dex), and successfully induced differentiation into hepatocyte-like cells after 14 days. Testing for the expression of albumin (ALB), α-fetoprotein (AFP) and cytokeratin 18 (CK18) and the accumulation of glycogen, which are unique markers for metabolically active hepatocytes, we proved that BMSCs could indeed differentiate specifically into mature hepatocytes. The albumin marker in stem cells was studied in several reports. Fang et al. observed albumin-positive donor-derived cells at a lower frequency in CCl4-damaged liver tissue [24]. Some other studies reported a marked increase in the expression of albumin after 4 weeks of BMSC transplantation [25]. In our study, although we observed the increased levels of ALB expression in BMSCs after induction of hepatic differentiation in vitro, we found no statistically significant difference in the ALB expression in the induced BMSCs before and after transplantation (Data not shown). The reason for this might have been the low concentration of inducing factors.
To create an acute liver failure rat model for cell transplantation experiment, we first treated rats with different CCl4 dosages, and based on an overall assessment of the rats’ appearance, activity, liver function, and pathological changes, we selected an optimal dose. At 3 ml/kg of 50% CCl4, the resulting liver damage did not reach acute liver failure levels, but at 5 ml/kg of 50% CCl4, the death rate was too high. Thus we chose a one-time intraperitoneal injection of 50% CCl4 at 4 ml/kg for the establishment of the rat acute liver failure model, and were able to better simulate the pathological and physiological changes of acute liver failure while achieving good stability. This was the same CCl4 dosage used by Taniguchi et al. to establish an acute liver failure model in rats [26].
Some previous researcher reported, that BMSCs can repair CCl4-damaged liver by reducing inflammation, collagen deposition, and remodeling [27]. Sakaida et al. [28] found that BMSC treatment in 4-week CCl4-induced rats resulted in significantly reduced liver fibrosis. Furthermore, in our study, when induced or uninduced BMSCs were used to treat acute liver failure in rats, both groups showed similarly good therapeutic effects. The recovery of ALT, AST, and TBIL levels in the blood was significantly better in the transplantation groups than in the control group and there was still significant reduction 7 days after transplantation. Between the induced and uninduced BMSC groups, however, only the levels of albumin elevation was significantly different, while there was no significant difference in the other biochemical and pathological indices. It appears that the induced hepatocyte-like cells have no therapeutic advantage over the uninduced BMSCs in the liver failure model rats. However, possible reasons for this could be that the number of BMSCs induced to differentiate into hepatocyte-like cells was too low, or that the ability of the differentiated cells to localize to the liver was impaired. Further research is required to provide a definitive conclusion. However, DAPI staining showed that transplanted BMSCs did localize in the liver at day 3 after transplantation and kept in the liver until day 14 to a lesser extent, which might have been due to DAPI photobleaching, indicating that the tail vein injection method is appropriate, but two animals died after the transplantation, possibly due to pulmonary embolisms.
The condition of our BMSC transplantation must be improved by optimizing the number of cells to deliver, the speed of injection, or a delivery through the portal vein.
In conclusion, bone marrow mesenchymal stem cells were induced to differentiate into hepatocyte like cells in vitro. The mRNA levels of the unique hepatocyte markers CK18 and ALB, and the level of glycogen, along with Alb protein expression, increased over time after induction, while the AFP mRNA level decreased. Together with the histological observation, the result demonstrated that induced BMSCs differentiated into round-shaped hepatocyte-like cells, confirming the validity of our induction condition. Furthermore, we showed that transplanted BMSCs were able to assist liver recovery in rats with CCl4-induced acute liver failure. However, induced hepatocyte-like cells did not produce better therapeutic outcomes than un-induced BMSCs.
Acknowledgements
This study was supported by the Key project of Fujian Province Science and Technology Plan (2011Y0043) and The Medical Science and Technology Innovating Foundation of Nanjing Military Command of Chinese PLA (No. 2007534).
Disclosure of conflict of interest
None.
References
- 1.Yang Y, Tao C, Zhao D, Li F, Zhao W, Wu H. EMF acts on rat bone marrow mesenchymal stem cells to promote differentiation to osteoblasts and to inhibit differentiation to adipocytes. Bioelectromagnetics. 2010;31:277–285. doi: 10.1002/bem.20560. [DOI] [PubMed] [Google Scholar]
- 2.Allan EH, Ho PW, Umezawa A, Hata J, Makishima F, Gillespie MT, Martin TJ. Differentiation potential of a mouse bone marrow stromal cell line. J Cell Biochem. 2003;90:158–169. doi: 10.1002/jcb.10614. [DOI] [PubMed] [Google Scholar]
- 3.Xue JX, Gong YY, Zhou GD, Liu W, Cao Y, Zhang WJ. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells induced by acellular cartilage sheets. Biomaterials. 2012;33:5832–5840. doi: 10.1016/j.biomaterials.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Dong XJ, Zhang GR, Shao JZ, Xiang LX. In vitro differentiation of mouse bone marrow stromal stem cells into hepatocytes induced by conditioned culture medium of hepatocytes. J Cell Biochem. 2007;102:52–63. doi: 10.1002/jcb.21275. [DOI] [PubMed] [Google Scholar]
- 5.Anzalone R, Lo Iacono M, Corrao S, Magno F, Loria T, Cappello F, Zummo G, Farina F, La Rocca G. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010;19:423–438. doi: 10.1089/scd.2009.0299. [DOI] [PubMed] [Google Scholar]
- 6.Chivu M, Dima SO, Stancu CI, Dobrea C, Uscatescu V, Necula LG, Bleotu C, Tanase C, Albulescu R, Ardeleanu C, Popescu I. In vitro hepatic differentiation of human bone marrow mesenchymal stem cells under differential exposure to liver-specific factors. Transl Res. 2009;154:122–132. doi: 10.1016/j.trsl.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 8.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Montini E, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. Kinetics of liver repopulation after bone marrow transplantation. Am J Pathol. 2002;161:565–574. doi: 10.1016/S0002-9440(10)64212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Defrances MC, Wolf HK, Michalopoulos GK, Zarnegar R. The presence of hepatocyte growth factor in the developing rat. Development. 1992;116:387–395. doi: 10.1242/dev.116.2.387. [DOI] [PubMed] [Google Scholar]
- 11.Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, De Vito P, Baldini PM, Prat M, Di Nardo P. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 12.Wang PP, Wang JH, Yan ZP, Hu MY, Lau GK, Fan ST, Luk JM. Expression of hepatocyte-like phenotypes in bone marrow stromal cells after HGF induction. Biochem Biophys Res Commun. 2004;320:712–716. doi: 10.1016/j.bbrc.2004.05.213. [DOI] [PubMed] [Google Scholar]
- 13.Abdul Rahman H, Manzor NF, Tan GC, Tan AE, Chua KH. Upregulation of SOX-2, FZD9, Nestin, OCT-4 and FGF-4 expression in human chorion derived-stem cells after angiogenic induction. Med J Malaysia. 2008;63(Suppl A):57–58. [PubMed] [Google Scholar]
- 14.Bialas M, Krupka M, Janeczek A, Rozwadowska N, Fraczek M, Kotlinowski J, Kucharzewska P, Lackowska B, Kurpisz M. Transient and stable transfections of mouse myoblasts with genes coding for pro-angiogenic factors. J Physiol Pharmacol. 2011;62:219–228. [PubMed] [Google Scholar]
- 15.Xia CS, Zuo AJ, Wang CY, Wang YZ. Isolation of rabbit bone marrow mesenchymal stem cells using density gradient centrifugation and adherence screening methods. Minerva Med. 2013;104:519–525. [PubMed] [Google Scholar]
- 16.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 17.Gruttadauria S, Grosso G, Pagano D, Biondi A, Echeverri GJ, Seria E, Pietrosi G, Liotta R, Basile F, Gridelli B. Marrow-derived mesenchymal stem cells restore biochemical markers of acute liver injury in experimental model. Transplant Proc. 2013;45:480–486. doi: 10.1016/j.transproceed.2012.06.087. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Hirai M, Cantero S, Ciubotariu R, Dobrila L, Hirsh A, Igura K, Satoh H, Yokomi I, Nishimura T, Yamaguchi S, Yoshimura K, Rubinstein P, Takahashi TA. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 2011;112:1206–1218. doi: 10.1002/jcb.23042. [DOI] [PubMed] [Google Scholar]
- 19.Solchaga LA, Penick KJ, Welter JF. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol Biol. 2011;698:253–278. doi: 10.1007/978-1-60761-999-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu S, De Becker A, Van Camp B, Vanderkerken K, Van Riet I. An improved harvest and in vitro expansion protocol for murine bone marrow-derived mesenchymal stem cells. J Biomed Biotechnol. 2010;2010:105940. doi: 10.1155/2010/105940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo JQ, Gao X, Lin ZJ, Wu WZ, Huang LH, Dong HY, Chen J, Lu J, Fu YF, Wang J, Ma YJ, Chen XW, Wu ZX, He FQ, Yang SL, Liao LM, Zheng F, Tan JM. BMSCs reduce rat granulosa cell apoptosis induced by cisplatin and perimenopause. BMC Cell Biol. 2013;14:18. doi: 10.1186/1471-2121-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 23.Kikkawa R, Fujikawa M, Yamamoto T, Hamada Y, Yamada H, Horii I. In vivo hepatotoxicity study of rats in comparison with in vitro hepatotoxicity screening system. J Toxicol Sci. 2006;31:23–34. doi: 10.2131/jts.31.23. [DOI] [PubMed] [Google Scholar]
- 24.Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83–88. doi: 10.1097/01.tp.0000128326.95294.14. [DOI] [PubMed] [Google Scholar]
- 25.Ali G, Masoud MS. Bone marrow cells ameliorate liver fibrosis and express albumin after transplantation in CCl(4)-induced fibrotic liver. Saudi J Gastroenterol. 2012;18:263–267. doi: 10.4103/1319-3767.98433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi M, Takeuchi T, Nakatsuka R, Watanabe T, Sato K. Molecular process in acute liver injury and regeneration induced by carbon tetrachloride. Life Sci. 2004;75:1539–1549. doi: 10.1016/j.lfs.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 27.Zhao L, Feng Z, Hu B, Chi X, Jiao S. Ex vivo-expanded bone marrow mesenchymal stem cells facilitate recovery from chemically induced acute liver damage. Hepatogastroenterology. 2012;59:2389–2394. doi: 10.5754/hge12288. [DOI] [PubMed] [Google Scholar]
- 28.Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]


