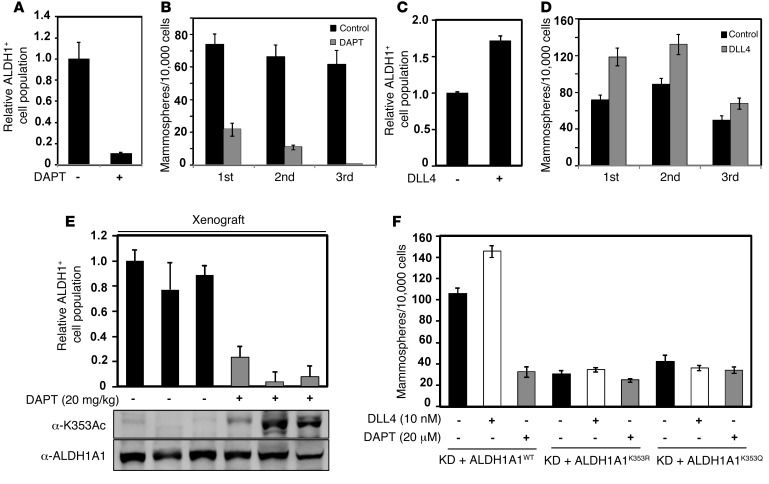
Figure 7. Mutation of the K353 acetylation site in ALDH1A1 inhibits NOTCH signaling to promote breast CSCs.
(A and B) Inhibition of NOTCH signaling reduced ALDH1+ cell populations and mammosphere formation. MDA-MB-468 cells were treated with 20 μM DAPT, followed by ALDEFLUOR assay (A) and mammosphere-forming assay (B). (C and D) Activation of the NOTCH signaling pathway promoted breast CSCs. MDA-MB-468 cells were treated with 10 nM DLL4, followed by ALDEFLUOR assay (C) and mammosphere-forming assay (D). (E) NOTCH inhibitor treatment enhanced K353 acetylation and decreased ALDH1+ cell populations in vivo. Xenografting into mammary fat pads was performed using 104 MDA-MB-468 cells, and xenograft tumors were treated with DAPT (20 mg/kg/mouse) every week. After 6 weeks, ALDH1A1, K353 acetylation, and ALDH1+ cell populations in tumors were analyzed. (F) K353R/Q mutation blocked the effect of the NOTCH signaling pathway on mammosphere formation. MDA-MB-468 cells reexpressing ALDH1A1WT, ALDH1A1K353R, and ALDH1A1K353Q mutants were treated with DAPT or DLL4 at the indicated concentrations, and mammosphere formation was measured. Data represent the mean ± SD of triplicate experiments for relative ALDH1+ cell populations and number of mammospheres per 10,000 transplanted cells.