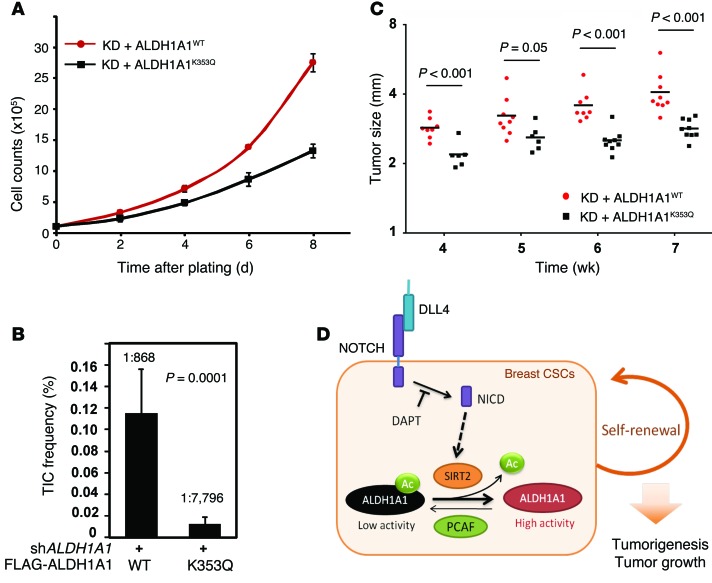
Figure 8. Lys-353 acetylation of ALDH1A1 inhibits breast tumorigenesis and tumor growth.
(A) ALDH1A1K353Q was compromised to support breast cancer cell proliferation. MDA-MB-468 cells with stably knocked down ALDH1A1 and reintroduced ALDH1A1WT or ALDH1A1K353Q were seeded into each well. Cell numbers were counted every 48 hours (error bars represent the mean ± SD of triplicate experiments). (B) MDA-MB-468 cells expressing ALDH1A1K353Q showed reduced TIC frequencies. Xenografting into mammary fat pads was performed using different concentrations (5 × 105, 5 × 104, 5 × 103, 1 × 103, 200, and 40 cells per mouse) of endogenous ALDH1A1-knockdown MDA-MB-468 cells that expressed either ALDH1A1WT or ALDH1A1K353Q. After 3 weeks, their tumorigenesis was determined, and TIC frequencies were calculated with L-Calc software. (C) ALDH1A1K353Q was defective in supporting tumor growth in vivo. Tumor sizes from the mouse injected with 5 × 105, 5 × 104, and 5 × 103 cells were monitored every week after tumor formation. Tumor growth derived from the mice injected with 5 × 103 cells is shown. (B and C) P values were calculated by Student’s t test. (D) Schematic illustration of NOTCH-mediated ALDH1A1 K353 deacetylation in the promotion of breast CSCs. High ALDH1A1 activity is required to maintain breast CSCs. K353 acetylation reduces ALDH1A1 enzyme activity, thereby inhibiting self-renewal of breast CSCs. NOTCH signaling is elevated in breast CSCs, and NOTCH induces SIRT2 to deacetylate and activate ALDH1A1 to maintain breast CSCs. NICD, NOTCH intracellular domain; Ac, acetylation.