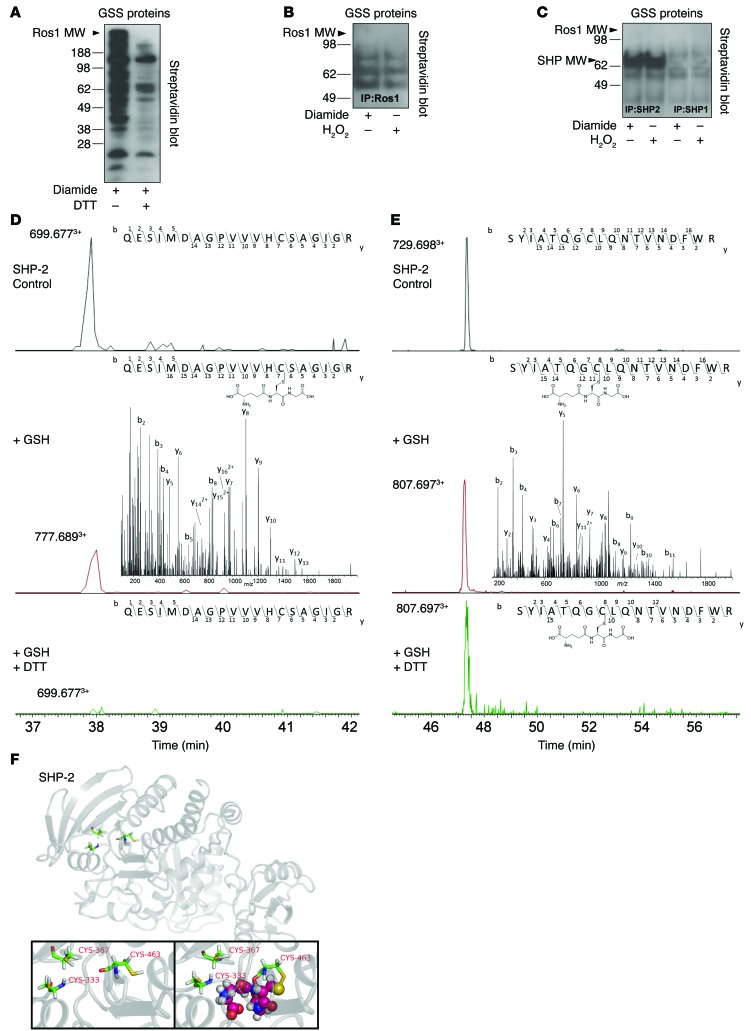
Figure 8. s-Glutathiolation of SHP-2 catalytic and backdoor cysteines inhibits ROS1 deactivation.
(A) HEK293 cells transfected with myc-tagged ROS1 and incubated with biotinylated GSH-ethyl-ester induced cysteine activation and s-glutathiolation of many proteins inhibitable by the reducing agent DTT. (B) s-Glutathiolation of ROS1 was not detected following immunoprecipitation; however, other GSS-protein conjugates were detected. Peptide mass fingerprinting showed that SHP-1 and SHP-2 were coimmunoprecipitated with ROS1, and (C) immunoprecipitation identified s-glutathiolation of SHP-2. LC-ESI-MS/MS identified the sites of glutathiolation. Extracted ion chromatograms of the SHP-2 peptides (D) Q450-R469 and (E) S326-R343 in the native form (top panel, control), s-glutathiolated form (middle panel), and reduced form (bottom panel) identify the catalytic Cys 463 and backdoor Cys 333 as the sites of s-glutathiolation. Insets are the representative MS/MS spectra of the Q450-R469 and S326-R343 peptide, providing site-specific localization of s-glutathiolation in the case of the modified forms. Upper insets represent the specific B and y ions as detected in the MS/MS spectra, showing inter-residue bond breakage generating b13 and y7 ions for Cys 463 and b8 and y11 for Cys 333, providing absolute confirmation. (F) (Upper panel and inset on left) Schematic representation of SHP-2 showing catalytic Cys 463 and backdoor Cys 333 and Cys 367 in the active PTP domain. (Inset on right) Modeling of s-glutathiolation of catalytic Cys 463 depicted by the spheres, demonstrating a physical presence within the active phosphatase domain of the enzyme.