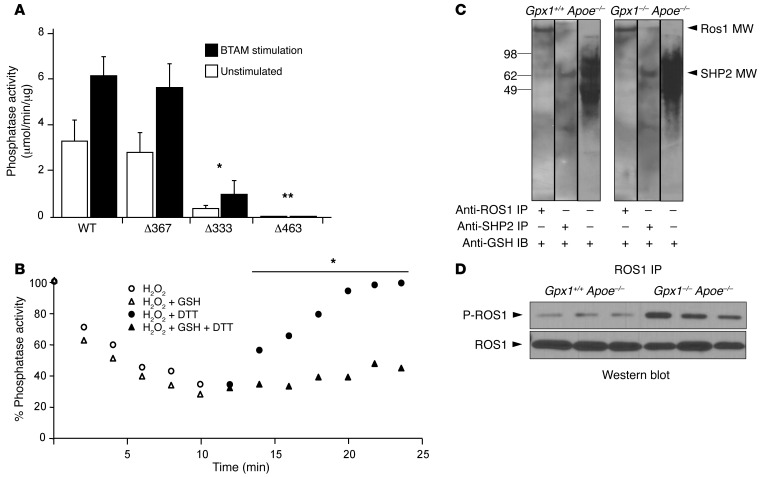
Figure 9. Catalytic and backdoor cysteines inhibit ROS1 deactivation in vitro and in vivo.
(A) Cys/Ser site-directed mutagenesis of catalytic Cys 463 and backdoor Cys 333 and Cys 367 were performed and phosphatase activity was assessed with and without stimulation. C367S did not affect phosphatase activity, whereas C333S led to a 90% reduction in phosphatase activity, and C463S abolished all activity compared with wild-type. *P < 0.05 compared with wild-type; **P < 0.05 compared with C333. (B) SHP-2 time-dependent inactivation by H2O2 and reactivation by DTT. Full-length SHP-2 was oxidized or glutathiolated and phosphatase activities measured in a continuous assay, after which DTT was added. Both oxidation and glutathiolation could induce deactivation of SHP-2; however, recovery following addition of the reducing agent DTT was significantly impaired in the glutathiolation group. *P < 0.05 compared with H2O2 + DTT. (C) SHP-2 is glutathiolated in vivo in experimental animals. Western blotting for glutathiolated proteins in vessel homogenate identified increased band intensity in Gpx1–/– Apoe–/– mice compared with Gpx1+/+ Apoe–/–. Balloon angioplastied vessels were homogenized and immunoprecipitated for ROS1 or SHP-2 under nonreducing conditions. The immunoprecipitated protein was immunoblotted with anti-GSH antibody. Blotting of immunoprecipitate identified the presence of glutathiolated SHP-2, but also ROS1, suggesting a physical interaction in vivo. (D) Increased ROS1 activity in Gpx1–/– Apoe–/– mice in vivo. Balloon angioplastied vessels harvested 28 days postprocedurally were assessed for Ros1 phosphorylation. Levels of ROS1 phosphorylation were higher in Gpx1–/– Apoe–/– mice (P < 0.05) without differences in ROS1 protein.