Abstract
The LIN-1 ETS transcription factor plays a pivotal role in controlling cell fate decisions during development of the Caenorhabditis elegans vulva. Prior to activation of the RTK/Ras/ERK-signaling pathway, LIN-1 functions as a SUMOylated transcriptional repressor that inhibits vulval cell fate. Here we demonstrate using the yeast two-hybrid system that SUMOylation of LIN-1 mediates interactions with a protein predicted to be involved in transcriptional repression: the RAD-26 Mi-2β/CHD4 component of the nucleosome remodeling and histone deacetylation (NuRD) transcriptional repression complex. Genetic studies indicated that rad-26 functions to inhibit vulval cell fates in worms. Using the yeast two-hybrid system, we showed that the EGL-27/MTA1 component of the NuRD complex binds the carboxy-terminus of LIN-1 independently of LIN-1 SUMOylation. EGL-27 also binds UBC-9, an enzyme involved in SUMOylation, and MEP-1, a zinc-finger protein previously shown to bind LIN-1. Genetic studies indicate that egl-27 inhibits vulval cell fates in worms. These results suggest that LIN-1 recruits multiple proteins that repress transcription via both the SUMOylated amino-terminus and the unSUMOylated carboxy-terminus. Assays in cultured cells showed that the carboxy-terminus of LIN-1 was converted to a potent transcriptional activator in response to active ERK. We propose a model in which LIN-1 recruits multiple transcriptional repressors to inhibit the 1° vulval cell fate, and phosphorylation by ERK converts LIN-1 to a transcriptional activator that promotes the 1° vulval cell fate.
Keywords: LIN-1, SUMO, ETS, chromatin, vulval development, transcription
CELL fates are established during development by spatially controlled activation of signal transduction pathways that regulate transcription factors. The Elk subfamily of ETS transcription factors are critical targets of the receptor tyrosine kinase (RTK)/Ras/extracellular-regulated kinase (ERK)-signaling pathway (Treisman 1994). This subfamily includes extensively characterized proteins from three divergent animals: vertebrate Elk-1, Drosophila Aop/Yan, and Caenorhabditis elegans LIN-1. The mechanisms that enable ETS proteins to switch cell fates in response to signaling pathway activation are not fully defined, and to address this important issue it is critical to elucidate the function of these proteins both prior to ERK activation and following phosphorylation by ERK. These issues have significant implications for human health, since ETS proteins contribute to human diseases including cancer (Dittmer 2003).
lin-1 has been extensively characterized during development of the hermaphrodite vulva, an epidermal structure used for egg laying and sperm entry (reviewed by Horvitz and Sternberg 1991; Greenwald 1997; Kornfeld 1997; Sternberg 2005; Sundaram 2013). In third-larval-stage hermaphrodites, six ventral epidermal blast cells called P3.p–P8.p (Pn.p cells) lie along the anterior–posterior axis. These Pn.p cells are an equivalence group, since each cell can adopt the 1° vulval cell fate (eight descendants), the 2° vulval cell fate (seven descendants), or the nonvulval 3° cell fate (two descendants). During vulval induction, the anchor cell of the somatic gonad secretes the LIN-3 ligand, an epidermal growth factor (EGF) homolog, thereby activating the LET-23/RTK on the adjacent P6.p cell (Aroian et al. 1990; Hill and Sternberg 1992) . Activated LET-23 recruits the SEM-5 adaptor (GRB2) and the LET-341/SOS-1 guanine nucleotide exchange factor (SOS), followed by sequential activation of the GTPase LET-60 (RAS) and the protein kinases LIN-45 (RAF), MEK-2 (MAPK kinase, or MEK), and MPK-1 (ERK) (Beitel et al. 1990; Han and Sternberg 1990; Clark et al. 1992; Han et al. 1993; Lackner et al. 1994; Wu and Han 1994; Kornfeld et al. 1995; Wu et al. 1995; Chang et al. 2000; Hsu et al. 2002). Activated MPK-1 phosphorylates substrates including the LIN-1 ETS transcription factor, thereby promoting the 1° fate in P6.p. P6.p signals P5.p and P7.p to adopt 2° fates by activating a lateral signal involving LIN-12/Notch. The 22 descendants of P5.p, P6.p, and P7.p invaginate and differentiate to form the vulval structure. P3.p, P4.p, and P8.p receive neither signal and therefore adopt 3° fates. Mutations that reduce activation of RTK/Ras/MAPK signaling cause a vulvaless (Vul) phenotype, whereas mutations that constitutively activate this pathway cause more than three Pn.p cells to adopt the 1° or 2° vulval cell fate, resulting in a multivulval (Muv) phenotype characterized by ectopic patches of vulval tissue.
LIN-1 is a transcription factor that plays a critical role in establishing vulval cell fates. Loss-of-function mutations that abrogate sequence-specific DNA-binding activity cause a strong Muv phenotype, demonstrating that DNA binding is necessary for lin-1 function and that lin-1 function is necessary to inhibit the 1° fate (Sulston and Horvitz 1981; Beitel et al. 1995; Miley et al. 2004). Genetic epistasis studies established that lin-1 functions downstream of mpk-1 ERK (Ferguson et al. 1987; Lackner et al. 1994; Wu and Han 1994). LIN-1 contains 17 S/TP motifs that are potential ERK phosphorylation sites and two docking sites for ERK, the D-domain and the FQFP motif (Jacobs et al. 1998, 1999; Tan et al. 1998). Mutations of LIN-1 that decrease phosphorylation by ERK cause a gain-of-function Vul phenotype, indicating that phosphorylation is necessary to stop LIN-1 from inhibiting the 1° fate (Jacobs et al. 1998). These studies are consistent with two possible models. Phosphorylation by ERK may abrogate LIN-1 activity in P6.p. Alternatively, phosphorylation may convert LIN-1 from an inhibitor to an activator of the 1° cell fate. Here we address these models by analyzing how phosphorylation by ERK affects the transcriptional activity of LIN-1. Our results indicate that the carboxy-terminus of LIN-1 is converted from a transcriptional repression domain to a potent transcriptional activation domain by ERK phosphorylation.
LIN-1 contains two consensus SUMOylation motifs. The E2 small ubiquitin-related modifier (SUMO) conjugating enzyme, UBC-9, binds these motifs and mediates the covalent attachment of SUMO to LIN-1 (Leight et al. 2005). C. elegans smo-1, the gene encoding SUMO, and ubc-9 inhibit vulval cell fates (Poulin et al. 2005) and genetically interact with lin-1 (Leight et al. 2005), indicating that SUMOylation of LIN-1 mediates inhibition of vulval cell fates. SUMOylation of LIN-1 promotes transcriptional repression (Leight et al. 2005), but the mechanism has not been well defined. We previously showed that SUMOylated LIN-1 interacts with MEP-1, a component of the NuRD transcriptional repression complex, suggesting that this interaction is one mechanism of LIN-1 transcriptional repression (Leight et al. 2005). The HOX gene lin-39 is a positive regulator of vulval cell fates and a direct transcriptional target of LIN-1 (Wagmaister et al. 2006). Guerry et al. (2007) showed that LIN-1 interacts with the Mi-2 nucleosome-remodeling protein LET-418 on the lin-39 promoter.
Here we identify proteins that bind LIN-1 and may contribute to transcriptional repression by LIN-1. SUMOylation of LIN-1 mediated recruitment of two chromatin regulatory enzymes in yeast cells: SET-6 and RAD-26. SET-6 is homologous to SUV39H1, a histone methyltransferase that mediates transcriptional repression (Bannister et al. 2001; Lachner et al. 2001; Nielsen et al. 2001). RAD-26 shares homology with the Mi-2β/CHD4 component of the NuRD transcriptional repression complex. In addition, the EGL-27/MTA1 component of the NuRD complex binds the carboxy-terminus of LIN-1 in yeast cells independently of SUMOylation. These findings suggest that LIN-1 recruits cofactors via both its SUMOylated amino-terminus and its unSUMOylated carboxy-terminus to inhibit vulval cell fates. rad-26 and egl-27 function to inhibit vulval cell fates in worms. These data support a model in which SUMOylated LIN-1 recruits multiple chromatin regulatory proteins to repress transcription of vulval cell-fate genes, and phosphorylation by ERK MAP kinase in the P6.p cell converts LIN-1 to a transcriptional activator that promotes vulval cell fates.
Materials and Methods
Yeast two-hybrid screens and reporter gene assays
The YEL3 strain encoding a LexADBD:LIN-1(1–252) fusion protein was described by Leight et al. (2005). The YEL2 strain was generated by transforming the L40 strain (Vojtek et al. 1993) with a bait plasmid encoding LIN-1(253–441) cloned into pBTM116 (Bartel et al. 1993). A random-primed complementary DNA (cDNA) library from mixed-stage hermaphrodites (kindly provided by R. Barstead), containing cDNAs fused to the GAL4AD, was transformed into YEL2 and YEL3 (Schiestl and Gietz 1989). Prey plasmids were isolated from positive colonies. cDNAs that failed to PCR-amplify with MEP-1 or MPK-1 gene-specific primers were sequenced using standard techniques.
Bait plasmids encoding EGL-27(1–1129), which corresponds to the largest predicted egl-27 transcript, EGL-27(1–637), and EGL-27(629–1129) (Herman et al. 1999; Solari et al. 1999), were cloned into pAS2-1 (CLONTECH, Palo Alto, CA) and transfected into Saccharomyces cerevisiae strain Hf7c (Feilotter et al. 1994). Positive clones growing on His dropout media were assayed for β-galactosidase activity.
To monitor activation of the LexA-dependent lacZ reporter, we prepared lysates from at least six independent yeast transformants of equivalent size and measured β-galactosidase activity using the Galacto-Light Plus System (Applied Biosystems). For Figure 2, yeast transformants were grown in selective media at 30° to an OD of ∼1.0 before analysis.
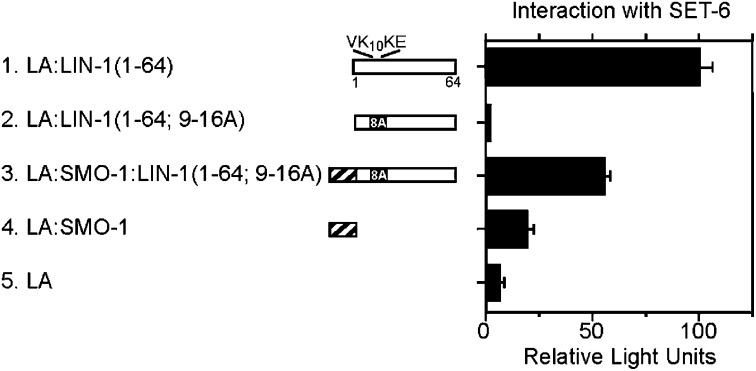
Figure 2.
SUMO was sufficient to increase binding of SET-6 to LIN-1 in yeast cells. Associations of SET-6 with LA fusion proteins were monitored using the yeast two-hybrid system. Bars represent the average and standard deviation of LexA-dependent β-galactosidase activity from three independent yeast transformants. Values were normalized by setting the interaction of SET-6 with LA:LIN-1(1–64) to 100. The LA fusion proteins were expressed at similar levels as determined by Western blotting (data not shown).
Analysis of C. elegans strains
C. elegans strains were cultured at 20° as described by Brenner (1974) unless otherwise noted. The following mutations were analyzed: let-60(n1046) (Beitel et al. 1990), lin-1(e1275 R175Opal) (Beitel et al. 1995), lin-1(n1790 R352Opal) (Jacobs et al. 1998), egl-27(n170) (Herman et al. 1999), egl-27(we3) (Solari et al. 1999), lin-15A(n767) and lin-15B(n744) (Ferguson and Horvitz 1989), mpk-1(oz140) (Lee et al. 2007), and set-6(tm1611) (Andersen and Horvitz 2007). rad-26(tm1991) was generated by the National Bioresource Project by screening animals mutagenized by trimethyl psoralen (TMP)/UV for a deletion of the C27B7.4 gene. We outcrossed tm1991 to the N2 wild-type strain twice and constructed double mutants using standard techniques. set-6(ok2195) was obtained from the Caenorhabditis Genetics Center (CGC) and outcrossed multiple times to the N2 wild-type strain.
cDNAs encoding RAD-26(17–655), SET-6(1–708) and EGL-27(691–1119) were cloned into the L4440 plasmid (Timmons and Fire 1998) for expression of double-stranded RNA (dsRNA). Plasmids were transformed into HT115(DE3) Escherichia coli. L4 hermaphrodites were placed on petri dishes containing bacteria that expressed dsRNA or control bacteria with the L4440 plasmid and transferred to new petri dishes daily; progeny were scored for the Muv phenotype.
Protein purification and GST pull-down assays
6xHis- and FLAG-tagged RAD-26(17–655) (HF:RAD-26) and SET-6(22–538) (HF:SET-6) were expressed in Sf9 cells using a baculovirus expression system (Invitrogen, Bac-to-Bac Baculovirus Expression Systems manual). DNA encoding EGL-27(691–1119) was cloned in a pET-28a(+) plasmid (EMD Biosciences) with the 6xHis- and T7 tag, and the protein was expressed in E. coli. HF:RAD-26, HF:SET-6, and T7:EGL-27 were purified using native conditions as described in Protocol 16 of the QIAexpressionist handbook (Qiagen, August 2002) with minor modifications. DNAs encoding LIN-1(1–64) and the SMO-1(1–88) were cloned in-frame with GST into the plasmid pGEX-4T-1 (Amersham Pharmacia Biotech). DNA encoding LIN-1(281–441) was cloned in-frame with GST in the plasmid pGEX-2T (Amersham Pharmacia Biotech). GST and GST fusion proteins were expressed in E. coli and partially purified under native conditions using glutathione sepharose as described (Jacobs et al. 1998).
For pull-down assays in Figure 3, equimolar amounts of HF:RAD-26 and HF:SET-6 were incubated with ∼5 μg of glutathione sepharose-bound GST, GST:LIN-1(1–64), or GST:SMO-1 for 1 hr at 4° in binding buffer (PBS + 0.1% NP-40). The glutathione sepharose was washed twice; bound proteins were eluted by boiling in sample buffer and separated by SDS-PAGE (Sambrook et al. 1989). HF:RAD-26 and HF:SET-6 were detected by immunoblotting using the α-FLAG M2 antibody (Sigma). Pull-down assays in Figure 5C were performed similarly, except T7:EGL-27 was incubated with ∼1 μg of glutathione sepharose-bound GST, GST:LIN-1(1–64), or GST:LIN-1(281–441). Bound T7:EGL-27 was detected by immunoblotting using the α-T7 Tag monoclonal antibody (EMD Biosciences). For pull-down assays in Figure 5D, the egl-27 cDNA was cloned into pGEX-4T-1 to encode GST:EGL-27(1–1129), and a full-length mep-1 cDNA was cloned into pTrcHis (Invitrogen) to encode 6xHis:MEP-1. Fusion proteins were expressed in E. coli strain BL21(DE3) and purified by the method of Frangioni and Neel (1993) with minor modifications. An equimolar amount of 6xHis:MEP-1 was added to GST and GST:EGL-27(1–1129) bound to glutathione sepharose beads. 6xHis:MEP-1 was detected with HRP-conjugated anti-histidine (anti-HIS) antibody (Santa Cruz) using the West Pico Super Signal Chemiluminescent detection system (Pierce).
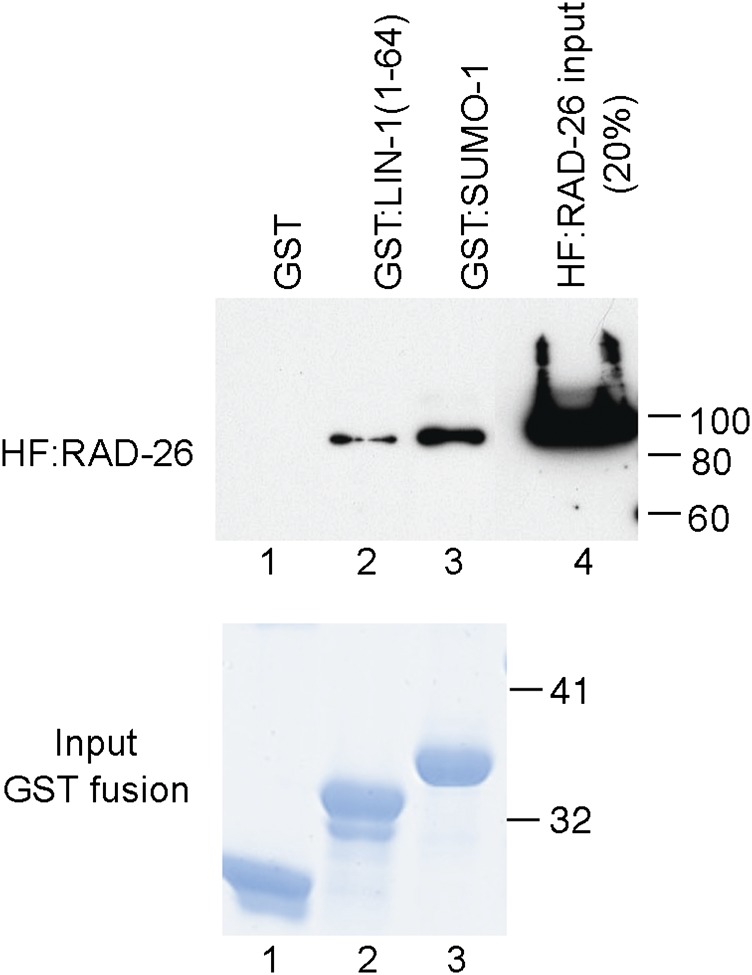
Figure 3.
RAD-26 binds SMO-1 and LIN-1 in purified extracts. HF:RAD-26(17–655) was incubated with equivalent amounts of glutathione agarose-bound GST, GST:LIN-1(1–64), or GST:SMO-1. Bound HF:RAD-26 was visualized by immunoblotting using α-FLAG antibody (top). Lane 4 shows 20% of the HF:RAD-26 input. HF:RAD-26 did not bind GST alone as assessed by prolonged exposure of the immunoblot. Input GST fusion proteins were visualized by SDS-PAGE and Simply Blue staining (bottom). Molecular weight markers (in kDa) are indicated.
Figure 5.
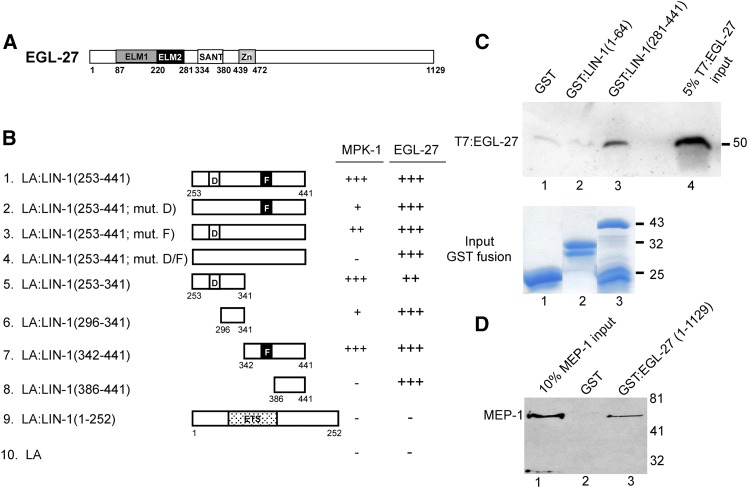
LIN-1 binds EGL-27 in yeast cells and purified extracts. (A) EGL-27 protein (Solari et al. 1999) contains ELM1 (dark shading), ELM2 (solid), SANT (open), and GATA-like zinc-finger (light shading) domains. (B) The interactions between LA:LIN-1 proteins and MPK-1(1–444) and EGL-27(691–1119) fused to the GAL4 activation domain were measured. Symbols indicate robust (+++), moderate (++), weak (+), and no detectable (−) activation of a LexA-dependent lacZ reporter gene. (C) 6xHis- and T7-tagged EGL-27(691–1119) (T7:EGL-27) was incubated with equivalent amounts of glutathione agarose-bound GST, GST:LIN-1(1–64) or GST:LIN-1(281–441). Bound T7:EGL-27 was visualized by immunoblotting using α-T7 antibody (top). Lane 4 shows 5% of the T7:EGL-27 input. Input GST fusion proteins were visualized by SDS-PAGE and Simply Blue staining (bottom). Lower-molecular-weight proteins in lanes 2 and 3 represent degradation products. (D) 6xHis-tagged MEP-1 (MEP-1) was incubated with equivalent amounts of glutathione agarose-bound GST or GST:EGL-27(1–1129). Bound MEP-1 was visualized by immunoblotting using anti-HIS antibody. Lane 1 shows 10% of the MEP-1 input.
Reporter gene assays in mammalian cultured cells
The MC3T3 mouse fibroblast cell line was grown in alpha-minimum essential medium (α-MEM) with 10% fetal calf serum and transfected as described by Fantz et al. (2001) with (1) the pFR-LUC reporter plasmid (Stratagene), (2) an expression plasmid that encodes GAL4 DNA-binding domain (G4) alone or fused to fragments of LIN-1 or Elk-1 (pFA-Elk, Stratagene), and (3) the CMV-β-galactosidase control plasmid. Cells were treated with 5% glycerol 1 day after transfection, incubated an additional 2 days, and harvested for cell lysates; luciferase and β-galactosidase activity were quantified. Cells were exposed to 50 μg/ml recombinant basic fibroblast growth factor (bFGF) (Upstate Biotechnology) for 20 hr beginning 2 days post-transfection. Site-directed mutagenesis was used to generate mut. D (K280A, K283A, L287A, L289A), mut. F (F381A, Q382A, F383A), and the double mutant (mut. D/F).
Results
LIN-1 interacted with RAD-26, SET-6, and EGL-27 in yeast cells
To identify proteins that associate with LIN-1 (Figure 1A), we conducted yeast two-hybrid screens. In the first screen, the bait was the LexA DNA-binding domain (LA) fused to the amino-terminal 252 residues of LIN-1, which includes the ETS DNA-binding domain and two consensus SUMOylation motifs. A total of 233 cDNAs that encode proteins that bind LIN-1(1–252) specifically were identified by screening 4 × 106 cDNAs from a mixed-stage C. elegans cDNA library. A total of 123 cDNAs encoded the zinc-finger protein MEP-1 that we described previously (Leight et al. 2005). Four cDNAs derived from the C27B7.4/rad-26 gene. The predicted RAD-26 protein contains a DNA-dependent ATPase domain of the SNF2 family N-terminal class and a helicase C-terminal domain (Figure 1B). RAD-26 shares 23% identity over 883 amino acids with LET-418/CHD-4, a component of the NuRD transcriptional repression complex. Interestingly, LET-418/CHD-4 binds MEP-1 (Unhavaithaya et al. 2002). Two cDNAs derived from the C49F5.2/set-6 gene. The predicted SET-6 protein contains a SET lysine methyltransferase domain and is predicted to function as a histone H3 lysine 9 methyltransferase based upon its homology to the transcriptional repressor SUV39H1 (Bannister et al. 2001; Lachner et al. 2001; Nielsen et al. 2001) (Figure 1B).
Figure 1.
LIN-1 binds RAD-26 and SET-6 in yeast cells. (A) Schematic of LIN-1 showing the ETS DNA-binding domain (dotted), consensus SUMOylation motifs (ψKxE), and docking sites for ERK MAP kinase [D-domain (open) and FQFP motif (solid)]. Amino acid numbers are shown below. (B) SET-6 contains a SET lysine methyltransferase domain (light shading). RAD-26 contains a DNA-dependent ATPase domain of the SNF2 family N-terminal class (open) and a helicase C-terminal domain (dark shading). (C) Interactions between LA DNA-binding domain:LIN-1 fusion proteins and SET-6(22–538) or RAD-26(17–655) fused to the GAL4 activation domain (GAL4AD) were measured qualitatively. Symbols indicate robust activation (+++) and no detectable activation (−) of a LexA-dependent lacZ reporter gene. (D and E) Interactions were measured quantitatively by assaying LexA-dependent β-galactosidase activity. Bars represent the average and standard deviation of three to six independent yeast transformants. Values were normalized by setting the signal with wild-type LA:LIN-1 to 100. (F) Expression of LA:LIN-1 proteins was analyzed by Western blotting using an anti-LA antibody. Lanes 1–14 correspond to proteins in columns 1–14 of D.
In the second screen, the bait was the LexA DNA-binding domain fused to the LIN-1 carboxy-terminus (residues 253–441), which includes the D-domain and FQFP docking sites for ERK MAP kinase. A total of 143 cDNAs that encode proteins that bind LIN-1(253–441) specifically were identified by screening 1.3 × 106 cDNAs. Thirty-seven cDNAs encoded MPK-1 ERK MAP kinase. Seven cDNAs encoded EGL-27. EGL-27 shares 24% identity and 41% similarity over 467 amino acids with the MTA-1 component of the NuRD transcriptional repression complex (Herman et al. 1999). EGL-27 contains an ELM1 domain that is predicted to mediate protein–protein interactions, an ELM2 domain that is predicted to mediate DNA binding or protein–protein interactions, a SANT DNA-binding domain, and a GATA zinc-finger domain.
Two SUMOylation motifs of LIN-1 are necessary to promote binding of RAD-26 and SET-6 in yeast cells
To define regions of LIN-1 that are sufficient to bind SET-6 and RAD-26, we analyzed fragments of LIN-1. LA:LIN-1(1–64) and LA:LIN-1(146–252) mediated robust binding of SET-6 and RAD-26, indicating that LIN-1 contains two separable binding sites for SET-6 and RAD-26 (Figure 1C). To identify amino acids necessary for binding, we analyzed mutants of LIN-1(1–64) with eight consecutive amino acids changed to alanine. Substitution of LIN-1 residues 9–16 dramatically reduced binding of SET-6 and RAD-26 by 106- and 44-fold, respectively (Figure 1D, line 3, and Figure 1F). LIN-1 contains a consensus SUMOylation motif in this region, VK10KE (Sampson et al. 2001). Substitution of the SUMO acceptor lysine (K10A) dramatically reduced binding of SET-6 and RAD-26 by 62- and 37-fold, respectively (Figure 1D, line 11). Substitution of the highly conserved glutamic acid (E12A) reduced binding of SET-6 and RAD-26 by 40- and 30-fold, respectively (Figure 1D, line 13). Substitution of the moderately conserved valine (V9A) decreased binding of SET-6 and RAD-26 by 6- and 5-fold, respectively, whereas mutation of the nonconserved lysine (K11A) had minimal effects (Figure 1D, lines 10 and 12). These results demonstrate a correlation between the function of each residue in the ψKxE motif in promoting SUMOylation and in promoting binding of SET-6 and RAD-26.
To investigate SET-6 and RAD-26 binding to LIN-1 residues 146–252, we analyzed the LIN-1(156–180) fragment that contains the VK169DE SUMOylation motif. SET-6 and RAD-26 robustly bound LIN-1(156–180) (Figure 1E). Mutations of the entire motif (168–171A) or the predicted SUMO acceptor lysine (K169A) markedly reduced binding of LIN-1 to SET-6 and RAD-26 (Figure 1E). Thus, 64 residues of LIN-1 containing the SUMOylation motif VK10KE and 25 residues of LIN-1 containing the SUMOylation motif VK169DE were sufficient to bind SET-6 and RAD-26, and for both LIN-1 fragments the SUMO acceptor lysine was necessary for binding.
SUMOylation of LIN-1 promotes binding of RAD-26 and SET-6 in yeast cells
The ψKxE consensus SUMOylation motifs of LIN-1 are post-translationally modified by SUMO-1 in yeast cells (Leight et al. 2005). If SUMOylation of the ψKxE motifs promotes binding of SET-6 and RAD-26, then the addition of SUMO-1 to a SUMOylation-defective LIN-1 mutant might restore binding. To mimic the SUMOylated isoform of LIN-1, we generated a translational fusion of C. elegans SMO-1 and the LIN-1(1–64; 9–16A) SUMOylation-defective mutant. This fusion protein functioned as efficiently as SUMOylated LIN-1(1–64) to repress transcription in cultured cells, indicating that this translational fusion of SMO-1 can be functional (Leight et al. 2005). The interaction of SET-6 and RAD-26 with LA:SMO-1:LIN-1(1–64; 9–16A) was increased by 24- and 3-fold, respectively (Figure 2, lines 2 and 3; data not shown). These results indicate that SET-6 and RAD-26 bind SUMOylated LIN-1.
RAD-26 directly binds SUMO and LIN-1
To determine if LIN-1 directly binds SET-6 and RAD-26, we expressed histidine- and FLAG-tagged SET-6(22–538) (HF:SET-6) and RAD-26(17–655) (HF:RAD-26) proteins in insect cells and purified these proteins by metal affinity chromatography. GST-tagged C. elegans SMO-1 and LIN-1(1–64) were expressed in E. coli and purified by glutathione affinity chromatography. Binding was monitored by the GST pull-down method. HF:SET-6 did not bind GST:SMO-1 nor GST:LIN-1(1–64), indicating that additional proteins may facilitate the interaction between SET-6 and LIN-1 in yeast cells or the appropriate pull-down conditions were not identified. HF:RAD-26 did bind GST:SMO-1 and GST:LIN-1(1–64) specifically (Figure 3). These findings demonstrate that RAD-26 can directly bind both SMO-1 and LIN-1.
rad-26 functions in worms to inhibit vulval cell fates
To investigate the possibility that these protein interactions are functionally significant in worms, we reduced the activity of rad-26 and set-6 and monitored vulval development in wild-type and sensitized genetic backgrounds. rad-26 and set-6 have been analyzed in genome-wide screens using RNA interference (RNAi), but phenotypes have not been reported (Piano et al. 2002; Kamath et al. 2003; Sonnichsen et al. 2005). Wild-type hermaphrodites exposed to rad-26 dsRNA did not display a Muv phenotype (Table 1, line 15). The rad-26(tm1991) allele is deleted for exon 6 and a portion of exon 7 and is predicted to encode a truncated protein of 412 amino acids that lacks half of the ATPase domain and the entire helicase domain, suggesting that the mutant protein has reduced activity. rad-26(tm1991) mutants grown at 15° and 20° did not display a Muv phenotype (Table 1, lines 2 and 6).
Table 1. Genetic analysis of rad-26.
| Genotypea | RNAib | % Muvc | nd |
|---|---|---|---|
| 1. Wild typee | NA | 0 | 1702 |
| 2. rad-26(tm1991)e | NA | 0.1 | 1424 |
| 3. lin-1(e1275)e | NA | 41 | 2271 |
| 4. lin-1(e1275) rad-26(tm1991)e | NA | 89 | 1238 |
| 5. Wild type | NA | 0 | 1002 |
| 6. rad-26(tm1991) | NA | 0 | 308 |
| 7. lin-1(n1790) | NA | 8 | 840 |
| 8. lin-1(n1790) rad-26(tm1991) | NA | 29 | 1592 |
| 9. rad-26(tm1991); set-6(ok2195) | NA | 0 | 2345 |
| 10. egl-27(we3); rad-26(tm1991) | NA | 0 | 1678 |
| 11. mpk-1(oz140); rad-26(tm1991) | NA | 0 | 1596 |
| 12. rad-26(tm1911); lin15A(n767) | NA | 0 | 1843 |
| 13. rad-26(tm1911); lin-15B(n744) | NA | 0 | 1688 |
| 14. Wild type | Control | 0 | 1556 |
| 15. Wild type | rad-26 | 0 | 1787 |
| 16. let-60(n1046)e | Control | 16 | 375 |
| 17. let-60(n1046)e | rad-26 | 85 | 932 |
| 18. lin-1(n1790) | Control | 3 | 153 |
| 19. lin-1(n1790) | rad-26 | 11 | 262 |
To facilitate comparisons, the following experiments (indicated by line numbers) were conducted in parallel: 1–4, 5–8, 14–15, 16–17, and 18–19. The difference between the percentage of the lin-1(n1790) Muv phenotype in line 7 and line 18 might reflect day-to-day variability or the difference between control RNAi bacteria and standard OP50 bacteria as a food source.
L4 hermaphrodites were fed E. coli containing a control plasmid or a plasmid that expresses dsRNA from the rad-26 gene, and the vulval phenotype of the progeny that grew on these dishes was scored. NA, not applicable.
Adult hermaphrodites were scored as Muv if they displayed one or more ventral protrusions displaced from the site of the vulva. Hermaphrodites were propagated at 20° unless otherwise indicated. The χ2 test was used to compare two values. The following comparisons (indicated by line numbers) were significantly different (P < 0.05): 1 and 3, 2 and 4, 3 and 4, 5 and 7, 6 and 8, 7 and 8, 16 and 17, 18 and 19.
n, number of hermaphrodites examined.
Hermaphrodites were propagated at 15° because lin-1(e1275) and let-60(n1046) cause heat-sensitive Muv phenotypes.
To analyze interactions of rad-26 with the EGFR/Ras/MAPK pathway, we utilized sensitized genetic backgrounds. let-60(n1046gf) is a gain-of-function mutation that causes a temperature-sensitive Muv phenotype (Beitel et al. 1990). When let-60(n1046gf) hermaphrodites were fed rad-26 dsRNA, the penetrance of the Muv phenotype increased significantly from 16 to 85% (Table 1, line 17), indicating that rad-26 inhibits vulval cell fates. mpk-1(oz140) is a partial loss-of-function mutation that affects ERK MAP kinase and inhibits vulval induction: rad-26(tm1991) did not cause a Muv phenotype in this genetic background (Table 1, line 11). lin-1(e1275 R175Opal) is a partial loss-of-function mutation that causes a temperature-sensitive Muv phenotype. A double mutant with rad-26(tm1991) increased the penetrance of the lin-1(e1275) Muv phenotype significantly from 41 to 89% (Table 1, line 4). The lin-1 Muv phenotype is caused by a transformation of the cell fates of P3.p, P4.p, and P8.p from nonvulval to vulval (Ferguson et al. 1987). Nomarski optics were used to confirm that the lin-1(e1275) rad-26(tm1991) double-mutant animals displayed ectopic vulval cell fates (Figure 4, A and B). These results demonstrate that rad-26 functions to inhibit vulval cell fates and are consistent with the model that rad-26 is positioned in the EGFR/Ras/MAPK pathway or a parallel signaling pathway.
Figure 4.
Ectopic vulval protrusions in rad-26 and egl-27 mutant animals. (A–D) DIC images of the mid-body region. The genotypes are lin-1(e1275) rad-26(tm1991) (A and B) and egl-27(we3); lin-1(n1790) (C and D). Arrows mark protruding ectopic vulval tissue. Magnification is ×400.
To investigate the position of the rad-26 gene in the Ras pathway, we analyzed a lin-1 gain-of-function mutation. lin-1(n1790gf R352Opal) causes a weak vulvaless phenotype and partially suppresses the Muv phenotype caused by activated let-60 ras; the LIN-1(1–351) protein lacks the FQFP ERK docking site and is partially resistant to negative regulation by MPK-1 ERK (Jacobs et al. 1999). The lin-1(n1790gf) allele also causes a low penetrance Muv phenotype because the lin-1 mRNA contains a premature stop codon and is subject to nonsense-mediated decay. If rad-26 functions upstream of lin-1 in a linear signaling pathway, then we predict that the rad-26 mutation will not significantly affect the lin-1(n1790) phenotype. By contrast, if rad-26 functions downstream or at the level of lin-1, then we predict that the rad-26 mutation will promote a Muv phenotype in the lin-1(n1790) background. Specifically, if RAD-26 is recruited to SUMOylated LIN-1(1–351) and contributes to LIN-1 function, then removing the RAD-26 protein is predicted to impair the function of LIN-1(1–351) as a constitutive inhibitor of vulval cell fates, resulting in a Muv phenotype. The lin-1(n1790gf) rad-26(tm1991) double-mutant hermaphrodites displayed a Muv phenotype that was 29% penetrant, significantly greater than the Muv phenotype of n1790 and tm1991 single-mutant animals (Table 1, line 8). Consistent with this observation, lin-1(n1790gf) hermaphrodites fed rad-26 dsRNA displayed a weak but significant enhancement of the Muv phenotype (Table 1, line 19). These findings indicate that the LIN-1(1–351) protein may require rad-26 function to inhibit vulval cell fates efficiently.
To analyze the function of set-6, we reduced the activity of set-6 by feeding dsRNA. Wild-type hermaphrodites exposed to set-6 dsRNA did not display obvious vulval defects (data not shown). The set-6(tm1611) and set-6(ok2195) deletion mutations did not cause an independent Muv phenotype (data not shown). By contrast to rad-26, set-6(lf) mutations did not appear to interact with the EGFR/Ras/MAPK pathway. The Muv phenotype of lin-1(e1275); set-6(ok2195) double-mutant animals was similar to single-mutant lin-1(e1275) animals (data not shown). The Muv phenotype of let-60(n1046); set-6(tm1611) and let-60(n1046); set-6(ok2195) double-mutant animals was similar to that of let-60(n1046) single-mutant animals (data not shown). These findings indicate that set-6(lf) mutations do not interact strongly with mutations that affect the EGFR/Ras/MAPK pathway. set-6(ok2195) did not cause a Muv phenotype in combination with rad-26(tm1991) (Table 1, line 9) or egl-27(we3) (Table 2, line 8). These results do not provide evidence that set-6 plays a significant functional role in lin-1-mediated inhibition of vulval cell fates and raise the possibility that that the interaction between SET-6 and LIN-1 proteins may reflect a role in a different tissue.
Table 2. Genetic analysis of egl-27.
| Genotypea | RNAi | % Muvb | n |
|---|---|---|---|
| 1. Wild type | NA | 0 | 1002 |
| 2. egl-27(n170) | NA | 1 | 954 |
| 3. egl-27(we3) | NA | 1 | 594 |
| 4. lin-1(n1790) | NA | 8 | 840 |
| 5. egl-27(we3); lin-1(n1790) | NA | 21 | 438 |
| 6. egl-27(we3); lin-15A(n767) | NA | 0 | 1831 |
| 7. egl-27(we3); lin-15B(n744) | NA | 0 | 1639 |
| 8. egl-27(we3); set-6(ok2195) | NA | 0 | 1522 |
| 9. Wild type | Control | 0 | 1134 |
| 10. Wild type | egl-27 | 1 | 851 |
| 11. let-60(n1046)c | Control | 27 | 1466 |
| 12. let-60(n1046)c | egl-27 | 72 | 363 |
| 13. lin-1(n1790) | Control | 3 | 524 |
| 14. lin-1(n1790) | egl-27 | 28 | 200 |
Columns are as described in Table 1.
To facilitate comparisons, the following experiments (indicated by line numbers) were conducted in parallel; 4–5, 9–10, 11–12, and 13–14. The difference between the percentage of the let-60(n1046) Muv phenotype shown in line 11 compared to that in line 16 of Table 1 may reflect day-to-day variability in temperature or humidity.
The χ2 test was used to compare two values. The following comparisons were significantly different (P < 0.05): 1 and 2, 1 and 3, 1 and 4, 3 and 5, 4 and 5, 9 and 10, 11 and 12, and 13 and 14.
Hermaphrodites were propagated and scored at 15°.
The synthetic multivulva (synMuv) pathway is composed of two major groups of genes called class A and class B (Ferguson and Horvitz 1989). Loss-of-function mutations in a single class A gene or a single class B gene do not cause an independent Muv phenotype, but a double-mutant animal containing any class A mutation and any class B mutation displays a strong Muv phenotype. Thus, the class A and class B synMuv genes appear to function redundantly to inhibit vulval cell fates. The synMuv genes appear to function non-cell-autonmously in the hyp7 hypodermal cell to inhibit the expression and secretion of the lin-3 ligand; in class AB double-mutant animals, ectopic secretion of lin-3 causes the Muv phenotype (Herman and Hedgecock 1990; Cui et al. 2006). Several genes associated with the NURD complex have been proposed to function in the synMuv pathway, including let-418 and egr-1/lin-40 (Solari and Ahringer 2000; von Zelewsky et al. 2000). Andersen and Horvitz (2007) analyzed set-6 for a role in the synMuv pathway; the set-6(tm1611) deletion allele did not cause a Muv phenotype in combination the class A synMuv gene lin-15A or the class B synMuv gene lin-15B, indicating that set-6 is not a synMuv gene. To investigate the possibility that rad-26 is a synMuv gene, we generated double-mutant animals with lin-15A or lin-15B mutations. rad-26(tm1991) did not cause a Muv phenotype in combination with either class A or class B synMuv mutations, indicating that rad-26 is not a synMuv gene (Table 1, lines 12 and 13).
EGL-27 binds two domains of LIN-1 that are separable from the SUMOylation motifs and ERK-docking sites
EGL-27 was identified based on an interaction with LIN-1(253–441), which contains the carboxy-terminus. To characterize the interaction with EGL-27, we used the yeast two-hybrid system to define domains of LIN-1 that are necessary and sufficient to mediate binding. We mutagenized one or both of the ERK-docking sites and monitored the association of these LIN-1 derivatives with EGL-27 and MPK-1. MPK-1 displayed reduced binding to LA:LIN-1(253–441; mut. D) and LA:LIN-1(253–441; mut. F) and failed to bind LA:LIN-1(253–441; mut. D/F) (Figure 5B, lines 2–4). By contrast, EGL-27 efficiently bound each of these LIN-1 derivatives, indicating that the ERK-docking sites in LIN-1 are not necessary for EGL-27 binding.
To define regions of LIN-1 that are sufficient to bind EGL-27, we monitored the association of EGL-27 to fragments of LIN-1. These studies demonstrated that EGL-27 binds two domains in LIN-1 contained in residues 296–341 and residues 386–441 (Figure 5B, lines 6 and 8).
To determine if EGL-27 can directly bind LIN-1, histidine- and T7-tagged EGL-27(691–1119) were expressed in E. coli, purified using metal affinity chromatography, and monitored for binding to purified GST:LIN-1(281–441) using a GST pull-down assay. EGL-27 reproducibly bound GST:LIN-1(281–441) more efficiently than GST:LIN-1(1–64) or GST alone (Figure 5C), demonstrating that EGL-27 can directly bind the carboxy-terminus of LIN-1.
To further characterize the function of EGL-27, we used the yeast two-hybrid system to identify C. elegans proteins that interact with EGL-27. EGL-27(1–1129) was used to screen 3.4 × 106 cDNAs, and eight positive cDNAs were identified; three of these cDNAs corresponded to MEP-1. EGL-27(629–1129) was used to screen 6.9 × 104 cDNAs, and 49 positive cDNAs were identified; 2 of these cDNAs corresponded to UBC-9. MEP-1 and UBC-9 were previously demonstrated to interact with LIN-1 and to function to inhibit vulval cell fates during development (Leight et al. 2005). A fragment of MEP-1-containing residues 364–870, which includes all seven zinc-finger domains, was sufficient to interact with full-length EGL-27 in yeast. In addition, MEP-1 residues 330–870 were sufficient to interact with the amino-terminal 637 residues of EGL-27 in yeast (data not shown). The interaction of MEP-1 with full-length EGL-27 was demonstrated to be a direct interaction by a GST-pull-down assay (Figure 5D).
egl-27 inhibits vulval cell fates
egl-27 loss-of-function mutations were first identified based on egg-laying defects (Trent et al. 1983). egl-27 mutations also cause defects in TL and TR cell polarity, migration of HSN cells and QL neuroblast descendants (Herman et al. 1999), and embryonic patterning (Solari et al. 1999). egl-27 has been demonstrated to inhibit vulval cell fates since egl-27 RNAi was reported to enhance the Muv phenotype of lin-37(n758) egr-1(RNAi) animals from 21 to 50% (Solari and Ahringer 2000). egl-27(n170) was reported to enhance the let-60(n1046) Muv phenotype, increasing the percentage of induced P3.p cells from 47 to 79%, induced P4.p cells from 46 to 74%, and induced P8.p cells from 28 to 62% (Chen and Han 2001a). We hypothesized that LIN-1 recruits EGL-27 to repress transcription of vulval cell-fate genes. To test this prediction, we reduced the function of egl-27 using loss-of-function mutations or RNAi and monitored vulval development in different genetic backgrounds. egl-27(we3) is a nonsense mutation that results in a truncated protein of 811 amino acids (Solari et al. 1999). egl-27(n170) is a deletion of at least the first eight exons of egl-27 and affects the full-length transcript, but not the smaller transcripts (Herman et al. 1999). Both egl-27(n170) and egl-27(we3) hermaphrodites displayed a very low penetrance Muv phenotype of 1% (Table 2, lines 2 and 3). Likewise, 1% of wild-type hermaphrodites exposed to egl-27 RNAi displayed a Muv phenotype (Table 2, line 10). These results indicate that egl-27 functions to inhibit vulval cell fates, but the effect is difficult to measure in an otherwise wild-type background.
To investigate the possibility that egl-27 functions in the synMuv pathway, we generated double-mutant animals containing egl-27(we3) and a mutation of the class A synMuv gene lin-15A or the class B synMuv gene lin-15B. These double-mutant animals did not display a Muv phenotype, indicating that egl-27 is not a synMuv gene (Table 2, lines 6 and 7). This is similar to a previous report showing that egl-27 RNAi did not cause a Muv phenotype in combination with lin-15A and caused only a low-penetrance Muv phenotype (5%) in combination with the synMuv B gene lin-37 (Solari and Ahringer 2000).
To investigate the possibility that egl-27 functions in the EGFR/Ras/MAPK pathway, we examined egl-27 function in sensitized genetic backgrounds. The penetrance of the let-60(n1046gf) Muv phenotype was significantly increased from 27 to 72% in hermaphrodites fed egl-27 dsRNA (Table 2, line 12), consistent with previous studies demonstrating that egl-27(n170) enhanced the frequency of vulval cell fates of P3.p, P4.p, and P8.p (Chen and Han 2001a). The egl-27(we3); lin-1(n1790gf) double-mutant animals displayed a Muv phenotype that was 21% penetrant, significantly greater than the Muv phenotype of n1790 and we3 single mutants (Table 2, line 5). An analysis using DIC optics confirmed that these double-mutant animals displayed ectopic protrusions of vulval tissue (Figure 4, C and D). Likewise, 28% of lin-1(n1790gf) hermaphrodites fed egl-27 dsRNA displayed a Muv phenotype, which was significantly higher than the 3% of lin-1(n1790gf) hermaphrodites fed control bacteria that displayed a Muv phenotype (Table 2, line 14). Thus, two independent methods of reducing egl-27 function promoted vulval cell fates in the presence of lin-1(n1790), indicating that this is a robust result although the effect is not fully penetrant. These results are consistent with the model that EGL-27 may promote the ability of gain-of-function LIN-1(1–351) protein to inhibit vulval cell fates. egl-27(we3) did not cause a Muv phenotype in combination with rad-26(tm1991) (Table 1, line 10) or set-6(ok2195) (Table 2, line 8).
Carboxy-terminus of LIN-1 functions as an ERK-dependent transcriptional activation domain and an ERK-independent transcriptional repression domain
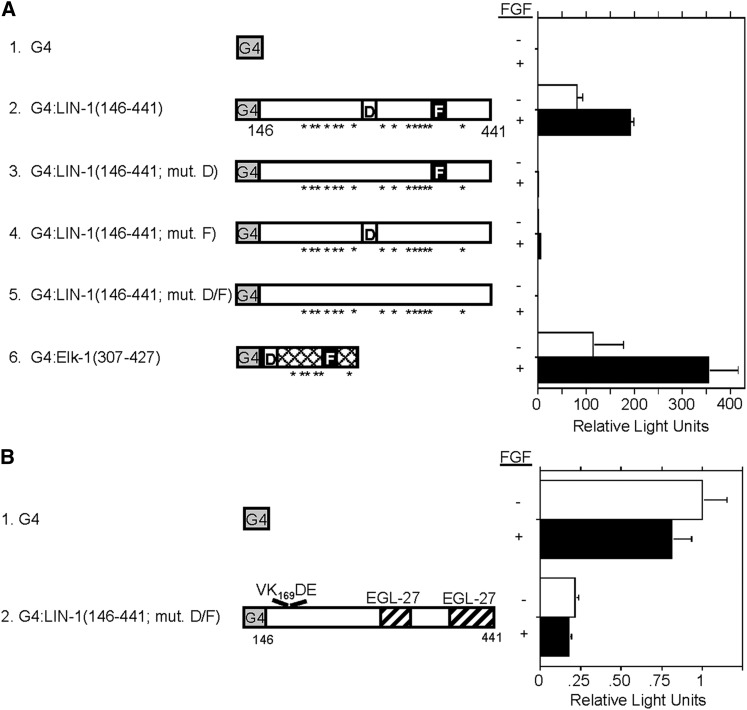
To analyze how phosphorylation by ERK regulates LIN-1, we analyzed transcriptional activity of the carboxy-terminus of LIN-1. Fragments of LIN-1 fused to the GAL4 DNA-binding domain were expressed in the MC3T3 murine fibroblast cell line, and transcription of a GAL4-dependent reporter gene was monitored. To increase ERK activity, we treated cells with bFGF. To decrease the ability of ERK to phosphorylate LIN-1, we mutated the ERK-docking sites (Jacobs et al. 1998, 1999). LIN-1(146–441) functioned as a potent activator of transcription, activating transcription by 81-fold relative to the G4 only control (Figure 6A, line 2). Treatment of cells with bFGF resulted in a twofold increase in the transcriptional activation potential of G4:LIN-1(146–441), suggesting that phosphorylation of LIN-1 by ERK promotes transcriptional activation. The activity of the LIN-1 carboxy-terminus in this assay is similar to the activity of the Elk-1 carboxy-terminus (Figure 6A, line 6). One possible explanation for the relatively small effect of adding bFGF is that ERK is already highly active under the standard culture conditions. This is likely since the cells were cultured with 10% fetal calf serum that contains a variety of growth factors that activate ERK.
Figure 6.
The carboxy-terminus of LIN-1 contains an ERK-dependent transcriptional activation domain and an ERK-independent repression domain. MC3T3 murine fibroblast cells were transfected with the following: the pFR-LUC reporter plasmid that contains five tandem GAL4-binding sites upstream of a basal promoter that regulates expression of luciferase; an expression plasmid that encodes GAL4 DNA-binding domain (G4) alone or fused to the indicated fragment of LIN-1 or Elk-1; and a reporter plasmid that encodes β-galactosidase to measure transfection efficiency. Cells were untreated (−) or treated with basic fibroblast growth factor (FGF) to activate ERK (+). (A) The schematics illustrate the ERK MAP kinase docking sites, the D-domain (D) and the FQFP motif (F). Asterisks indicate 15 S/TP motifs in LIN-1 that are potential ERK phosphorylation sites and 6 S/TP motifs in Elk-1 that are phosphorylated by ERK (Cruzalegui et al. 1999). Bars indicate luciferase activity divided by β-galactosidase activity. Values were normalized by setting the value for G4 alone in the absence of FGF to 1.0. Values represent the average and standard deviation of two to five independent transfections conducted in parallel. (B) The schematic of LIN-1 illustrates the VK169DE consensus SUMOylation motif (top) and two domains that are sufficient to bind EGL-27 (hatched).
To address the possibility that LIN-1 is highly phosphorylated by ERK under standard culture conditions, we investigated the effect of reducing phosphorylation of LIN-1 by mutating the docking sites for ERK. LIN-1 contains two docking sites for ERK, and we mutated only the D-domain, G4:LIN-1(146–441, mut. D); only the FQFP motif, G4:LIN-1(146–441, mut. F); and both the D-domain and the FQFP motif, G4:LIN-1(146–441, mut. D/F). Compared to wild-type LIN-1, these mutations reduced transcriptional activity by 251-, 75-, and 380-fold, respectively. These results indicate that, under standard culture conditions, binding of ERK to the D-domain and FQFP motif of LIN-1 and phosphorylation of LIN-1 dramatically increases the transcriptional activation potential of the carboxy-terminus of LIN-1.
These experiments allowed us to measure the transcriptional activity of the LIN-1 carboxy-terminus in the absence of ERK phosphorylation. The LIN-1(146–441) fragment contains one consensus SUMOylation motif and two binding sites for EGL-27. In the absence of ERK-docking sites, this fragment of LIN-1 repressed transcription by four- to sevenfold relative to G4 alone (Figure 6B, line 2). These results are consistent with the model that SUMOylation of LIN-1 and/or EGL-27 binding to the carboxy-terminus of LIN-1 mediates transcriptional repression and that phosphorylation by ERK converts LIN-1 to a transcriptional activator (Figure 7).
Figure 7.

ERK converts LIN-1 from a SUMOylated transcriptional repressor to a phosphorylated transcriptional activator. A model for the regulation of vulval cell fates by LIN-1. LIN-1 represses 1° vulval cell-fate genes by recruiting RAD-26 and MEP-1 to its SUMOylated amino-terminus and EGL-27 to its unSUMOylated carboxy-terminus. RAD-26 remodels nucleosomes, and EGL-27 and MEP-1 facilitate histone deacetylation through their association with HDAC-1 and HDAC-2. Following activation of the EGFR/Ras/MAPK pathway in P6.p, ERK converts LIN-1 to a phosphorylated transcriptional activator of 1° vulval cell-fate genes.
Discussion
SUMOylated LIN-1 recruits a nucleosome-remodeling enzyme to inhibit vulval cell fates
Genetic analyses demonstrate that lin-1 inhibits Pn.p cells from adopting vulval cell fates (Sulston and Horvitz 1981; Beitel et al. 1995; Jacobs et al. 1998). DNA binding and SUMOylation are necessary for this LIN-1 activity, suggesting that SUMOylated LIN-1 represses transcription of vulval cell-fate genes (Miley et al. 2004; Leight et al. 2005). Here we define a novel mechanism for transcriptional repression by SUMOylated LIN-1, recruitment of RAD-26.
We discovered RAD-26 as a LIN-1-interacting protein in yeast cells. SUMOylation of LIN-1 was necessary and sufficient to promote binding of RAD-26 in yeast cells. Furthermore, RAD-26 directly binds both LIN-1 and SUMO in purified extracts. These observations suggest that RAD-26 binds SUMOylated LIN-1 in worms; however, this biochemical interaction in worms has not been tested directly. Our genetic analyses demonstrated that rad-26 inhibits vulval cell fates. rad-26 interacted genetically with multiple genes in the Ras-signaling pathway that function in P6.p, and rad-26 did not cause a Muv phenotype in combination with class A or class B synMuv genes that function in hyp7. These genetic results are consistent with the model that rad-26 functions in the EGFR/Ras/MAPK-signaling pathway or that rad-26 functions in parallel to this signaling pathway, and they do not establish the cellular site of action of rad-26. Together, these biochemical and genetic results are consistent with the model that RAD-26 binding to LIN-1 promotes the ability of LIN-1 to repress primary vulval cell fates. Although reducing the activity of rad-26 or lin-1 causes ectopic vulval cell fates, the frequency of ectopic vulval cell fates is higher when lin-1 activity is reduced. This observation suggests that lin-1 retains some ability to inhibit vulval cell fates in the absence of rad-26.
RAD-26 is a homolog of vertebrate Mi-2β/CHD4, a core component of the NuRD transcriptional repression complex. This complex possesses ATP-dependent nucleosome-remodeling activity that is dependent upon Mi-2β/CHD4 and histone deacetylase (HDAC) activity provided by HDAC-1 and HDAC-2; both of these activities promote transcriptional silencing (Tong et al. 1998; Wade et al. 1998; Xue et al. 1998; Zhang et al. 1998; Wang and Zhang 2001). Multiple components of the C. elegans NuRD complex have been shown to inhibit vulval cell fates, including CHD-3 and LET-418 that are homologs of vertebrate Mi-2β/CHD4 (Lu and Horvitz 1998; Solari and Ahringer 2000; von Zelewsky et al. 2000; Chen and Han 2001a,b). Guerry et al. (2007) showed that LET-418 interacts with LIN-1 to repress transcription of the lin-39 target gene during vulval induction, although the role of SUMOylation was not described. Thus, LIN-1 interacts with two different homologs of vertebrate Mi-2: RAD-26 and LET-418. One possible explanation for the relatively mild vulval defects caused by rad-26(lf) mutations is that one or more genes function redundantly with rad-26. In particular, chd-3 and let-418 are highly similar to rad-26, and von Zelewsky et al. (2000) showed that let-418 and chd-3 function redundantly during vulval development. An important goal of future studies is to determine the unique and overlapping activities of rad-26, let-418, and chd-3. Together, these studies indicate that recruitment of the NuRD complex by LIN-1, and perhaps by additional transcription factors, is critical for inhibition of vulval cell fates.
The identification of RAD-26 provides important new insights into the mechanisms by which SUMOylated LIN-1 mediates transcriptional repression, since this protein has not been previously reported to interact with LIN-1 or contribute to vulval cell-fate specification. Furthermore, these findings have implications for other transcription factors. Over 30 transcription factors, including 7 ETS proteins, have been demonstrated to be post-translationally modified by SUMO-1 (Gill 2003; Hay 2005). In the majority of these cases, SUMOylation promotes repression of transcription. A critical question in this field is: How does SUMOylation mediate transcriptional repression? SUMOylation of p300 and Elk-1 promotes an association with histone HDACs that repress transcription (Girdwood et al. 2003; Yang and Sharrocks 2004). However, these HDACs were not shown to directly bind the SUMO moieties. Furthermore, the HDAC inhibitor trichostatin A (TSA) blocks repression at some, but not all, promoters (Yang et al. 2002). Thus, SUMO is likely to mediate transcriptional repression by additional, undefined mechanisms. In Drosophila cells, Mi-2, MEP-1, and a polycomb protein are important for SUMO-dependent transcriptional repression (Stielow et al. 2007). Our studies define a new mechanism by which SUMO mediates transcriptional repression: recruitment of a nucleosome-remodeling enzyme. Given that the NuRD complex possesses both nucleosome-remodeling and histone deacetylase activities, the direct binding of SUMO-1 to RAD-26/Mi-2β may account for the SUMO-1-mediated recruitment of HDAC activity observed in vertebrates (Girdwood et al. 2003; Yang and Sharrocks 2004).
EGL-27/MTA1 component of the NuRD complex binds LIN-1 independently of LIN-1 SUMOylation and inhibits vulval cell fates
We discovered EGL-27/MTA1 as a LIN-1-interacting protein in yeast cells. EGL-27 binds two domains in the carboxy-terminus of LIN-1 in yeast cells, and binding is independent of SUMOylation. Furthermore, EGL-27 directly binds LIN-1 in purified extracts. These results suggest that EGL-27 binds LIN-1 in worms; however, this biochemical interaction in worms has not been analyzed directly. The EGL-27 expression pattern was determined using an EGL-27::GFP reporter gene by Solari et al. (1999) and Herman et al. (1999). EGL-27 is expressed in somatic nuclei of most or all cells from the 50-cell stage of embryogenesis through adulthood. The results indicate that EGL-27 is expressed in the vulval precursor cells, which is consistent with the model that it interacts with LIN-1. EGL-27 is a homolog of the MTA1 component of the NuRD transcriptional repression complex, and we hypothesize that recruitment of EGL-27 to LIN-1 mediates transcriptional repression of vulval cell-fate genes. Consistent with this hypothesis, previous genetic studies showed that egl-27 inhibits vulval cell fates (Solari and Ahringer 2000; Chen and Han, 2001a). Our genetic analyses extend these findings by providing evidence that egl-27 interacts with multiple genes in the EGFR/Ras/MAPK-signaling pathway that function in Pn.p cells, including lin-1. egl-27 did not cause a Muv phenotype in combination with class A or class B synMuv genes that function in hyp7. These genetic results are consistent with the model that egl-27 functions in the Ras-signaling pathway or that egl-27 functions in parallel to the Ras-signaling pathway, and they do not establish the cellular site of action of egl-27. The Muv phenotype caused by reducing the activity of egl-27 is not as strong as the Muv phenotype caused by reducing the activity of lin-1, suggesting that lin-1 retains some function to inhibit vulval cell fates in the absence of egl-27. One possible explanation for the relatively mild vulval defects caused by egl-27(lf) mutations is that one or more genes function redundantly with egl-27. In particular, lin-40/egr-1 is highly similar to egl-27. Notably, Chen and Han (2001a,b) showed that lin-40 negatively regulates vulval cell-fate specification, and Solari et al. (1999) showed that egl-27 and lin-40/egr-1 function redundantly during embryonic patterning. An important goal of future studies is to determine the unique and overlapping activities of egl-27 and lin-40.
By searching for proteins that interact with EGL-27, we demonstrated that EGL-27 could interact with UBC-9 and MEP-1. UBC-9 is an E2 SUMO-conjugating enzyme that binds consensus SUMOylation motifs and mediates the covalent attachment of SUMO-1. EGL-27 contains five consensus SUMOylation motifs, including four in the EGL-27(629–1129) fragment that binds UBC-9, suggesting that EGL-27 may be SUMOylated. MEP-1 is a component of the NURD transcriptional repression complex (Unhavaithaya et al. 2002). We previously demonstrated that MEP-1 interacts with the SUMOylated N terminus of LIN-1 and functions to inhibit vulval cell fates (Leight et al. 2005). This interaction of MEP-1 with SUMO may be conserved since Drosophila MEP-1 mediates SUMO-dependent transcriptional repression (Stielow et al. 2007). These observations suggest that MEP-1 may be recruited by two mechanisms: directly by binding to the SUMOylated N terminus of LIN-1 and indirectly by binding to EGL-27.
Our studies demonstrate that LIN-1 binds multiple proteins that are predicted to function to repress transcription: RAD-26 and MEP-1 bind two SUMOylation motifs in the amino-terminus of LIN-1, and EGL-27 binds two domains in the carboxy-terminus of LIN-1. LIN-1 also binds the LIN-31 transcription factor (Tan et al. 1998) and LET-418 (Guerry et al. 2007). If these proteins function independently to modify chromatin and repress transcription, then elimination of one component might impair, but not abrogate, the function of the complex, resulting in a weak Muv phenotype. Mutations of rad-26, egl-27, and mep-1 all cause weak Muv phenotypes, consistent with the model that LIN-1 employs multiple independent mechanisms to repress transcription.
Phosphorylation of LIN-1 by ERK converts LIN-1 from a transcriptional repressor to a transcriptional activator
We examined how ERK affects the transcriptional activity of LIN-1 to determine if phosphorylation by ERK abrogates LIN-1 activity or converts LIN-1 to a transcriptional activator. If LIN-1 cannot be phosphorylated by ERK because the docking sites for ERK are mutated, then the carboxy-terminus of LIN-1 repressed transcription by about fivefold. By contrast, the carboxy-terminus of LIN-1 activated transcription by ∼80-fold when ERK-docking sites were intact. Stimulating ERK activity with bFGF caused a further 2-fold increase in transcriptional activation. Overall, the difference between transcriptional activation by LIN-1 that cannot be phosphorylated and LIN-1 that is maximally phosphorylated is ∼900-fold. The dramatic ERK-responsive transcriptional activation potential of the carboxy-terminus of LIN-1 strongly supports the model that ERK switches LIN-1 from a transcriptional repressor to a phosphorylated transcriptional activator.
These results extend the similarities between LIN-1 and vertebrate Elk-1, documenting the relevance of LIN-1 as a model for understanding vertebrate biology. Elk-1 was initially characterized as a ternary complex factor that binds the serum response element present in the promoters of immediate early genes, such as c-fos (Treisman 1994). Upon activation of the RTK/Ras/ERK pathway, ERK phosphorylates multiple sites in the carboxy-terminus of Elk-1, resulting in potent transcriptional activation of immediate early genes (Janknecht et al. 1993; Marais et al. 1993; Cruzalegui et al. 1999). By contrast, studies of the Drosophila ETS protein Aop/Yan indicated that, in the absence of activated ERK, Aop/Yan functions as an inhibitor of neuronal cell fates by mediating transcriptional repression (O’Neill et al. 1994). ERK-mediated phosphorylation of Aop/Yan results in its nuclear export and degradation, thus abrogating the repressor activity of Aop/Yan and allowing cells to adopt neuronal cell fates (Rebay and Rubin 1995). Our results indicate that LIN-1 regulation is different from that of Aop/Yan.
These molecular studies of LIN-1 provide a unifying hypothesis that explains diverse genetic observations of lin-1. In two instances where the EGFR/Ras/MAPK pathway functions during early development, lin-1 appears to be a positive mediator of this pathway. Howard and Sundaram (2002) showed that lin-1(lf) mutations could cause a larval lethal phenotype similar to loss-of-function mutations in positive regulators of EGFR/Ras/MAPK signaling, suggesting that lin-1 is a positive mediator of this signaling pathway during larval development. Jiang and Wu (2014) recently reported that the lin-3, let-23, let-60, mpk-1, lin-1 pathway promotes specific programmed cell-death events; phosphorylated LIN-1 directly binds the egl-1 promoter and activates transcription of egl-1, consistent with a transcriptional activation function of phosphorylated LIN-1. The function of lin-1 during vulval development is more complex. lin-1 null mutations cause P3.p, P4.p, and P8.p to adopt vulval cell fates, suggesting that lin-1 is necessary to inhibit vulval cell fates and may not be necessary to promote vulval cell fates (Sulston and Horvitz 1981; Ferguson et al. 1987). However, Tiensuu et al. (2005) showed that egl-17 expression in P6.p is abnormal in lin-1(lf) mutants, indicating that lin-1 function is required in P6.p for normal gene expression. Wagmaister et al. (2006) identified lin-39 as a directly regulated LIN-1 target gene. In lin-1(lf) mutants, lin-39 gene expression is upregulated in P3.p, P4.p, and P8.p, consistent with LIN-1 functioning as a transcriptional repressor in these cells. In addition, lin-39 gene expression is down-regulated in P6.p, consistent with LIN-1 functioning as a transcriptional activator in P6.p. Zhang and Greenwald (2011) demonstrated that lag-2, which encodes the ligand for Notch, is transcriptionaly repressed in all six Pn.p cells prior to ERK activation, and that this repression requires lin-1. Farooqui et al. (2012) proposed that nonphosphorylated LIN-1 directly binds and activates the let-502 promoter in the secondary cells P5.p and P7.p, suggesting that LIN-1 may have different regulatory mechanisms in the primary cell P6.p, the secondary cells P5.p and P7.p, and the tertiary cells P3.p, P4.p, and P8.p. We propose that SUMOylated LIN-1 represses transcription of target genes in P3.p, P4.p, and P8.p and that phosphorylated LIN-1 activates transcription of target genes in P6.p (Figure 7). In lin-1(lf) mutants, the absence of SUMOylated LIN-1 causes derepression of target genes in P3.p, P4.p, and P8.p, and these derepressed target genes are sufficient to promote a vulval cell fate. Although the absence of phosphorylated LIN-1 in P6.p results in diminished activation of target genes, P6.p can still adopt the proliferative vulval cell fate.
An important question that is raised by these findings is: How does phosphorylation by ERK convert LIN-1 from a transcriptional repressor to a transcriptional activator? Activation of ERK might result in decreased SUMOylation of LIN-1. For example, the steady-state level of SUMOylated Elk-1 decreases when ERK is activated in vertebrate cells (Yang et al. 2003). Phosphorylation of LIN-1 by ERK might disrupt the binding interaction of LIN-1 with transcriptional repressors. In particular, the EGL-27 transcriptional repressor binds two domains in the carboxy-terminus of LIN-1 that contain multiple consensus phosphorylation sites for ERK. Phosphorylated LIN-1 might recruit transcriptional activators. Phosphorylation of Ets-1 and Ets-2 by ERK mediates recruitment of the transcriptional coactivators CREB-binding protein and p300 (Foulds et al. 2004). Phosphorylated Elk-1 interacts with the mediator subunit Sur2 to promote transcriptional activation (Stevens et al. 2002; Cantin et al. 2003), and the C. elegans sur-2 gene promotes vulval cell fates (Singh and Han 1995), suggesting that phosphorylated LIN-1 might recruit SUR-2 to promote transcriptional activation. Proteins recruited by phosphorylated LIN-1 might reverse the chromatin modifications caused by transcriptional repressors, such as the histone deacetylation and nucleosome remodeling caused by the NuRD complex. These mechanisms are not mutually exclusive, and important goals of future studies are to determine the relative importance of these mechanisms and identify transcriptional activators recruited to LIN-1.
Acknowledgments
rad-26(tm1991) was provided by Shohei Mitani of the National Bioresource Project. We thank Tim Schedl, Kurt Warnhoff, and members of the Kornfeld laboratory for insightful comments on the manuscript. This research was supported by National Institutes of Health grants GM068598, CA84271 (to K.K.). K.K. was a Scholar of the Leukemia and Lymphoma Society and a Senior Scholar of the Ellison Medical Foundation. E. R. L. was supported by the W. M. Keck Postdoctoral Program in Molecular Medicine and a National Research Service Award from the National Institutes of Health (F32 GM66605).
Footnotes
Communicating editor: D. I. Greenstein
Literature Cited
- Andersen E. C., Horvitz H. R., 2007. Two C. elegans histone methyltransferases repress lin-3 EGF transcription to inhibit vulval development. Development 134: 2991–2999. [DOI] [PubMed] [Google Scholar]
- Aroian R. V., Koga M., Mendel J. E., Ohshima Y., Sternberg P. W., 1990. The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature 348: 693–699. [DOI] [PubMed] [Google Scholar]
- Bannister A. J., Zegerman P., Partridge J. F., Miska E. A., Thomas J. O., et al. , 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410: 120–124. [DOI] [PubMed] [Google Scholar]
- Bartel P. L., Chien C.-T., Sternglanz R., Fields S., 1993. Using the two-hybrid system to detect protein-protein interactions, p. 153 in Cellular Interactions in Development: A Practical Approach, edited by Hartley D. A. Oxford University Press, Oxford. [Google Scholar]
- Beitel G. J., Clark S. G., Horvitz H. R., 1990. Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature 348: 503–509. [DOI] [PubMed] [Google Scholar]
- Beitel G. J., Tuck S., Greenwald I., Horvitz H. R., 1995. The Caenorhabditis elegans gene lin-1 encodes an ETS-domain protein and defines a branch of the vulval induction pathway. Genes Dev. 9: 3149–3162. [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin G. T., Stevens J. L., Berk A. J., 2003. Activation domain-mediator interactions promote transcription preinitiation complex assembly on promoter DNA. Proc. Natl. Acad. Sci. USA 100: 12003–12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Hopper N. A., Sternberg P. W., 2000. Caenorhabditis elegans SOS-1 is necessary for multiple RAS-mediated developmental signals. EMBO J. 19: 3283–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Han M., 2001a Role of C. elegans lin-40 MTA in vulval fate specification and morphogenesis. Development 128: 4911–4921. [DOI] [PubMed] [Google Scholar]
- Chen Z., Han M., 2001b C. elegans Rb, NuRD, and Ras regulate lin-39-mediated cell fusion during vulval fate specification. Curr. Biol. 11: 1874–1879. [DOI] [PubMed] [Google Scholar]
- Clark S. G., Stern M. J., Horvitz H. R., 1992. C. elegans cell-signaling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature 356: 340–344. [DOI] [PubMed] [Google Scholar]
- Cruzalegui F. H., Cano E., Treisman R., 1999. ERK activation induces phosphorylation of Elk-1 at multiple S/T-P motifs to high stoichiometry. Oncogene 18: 7948–7957. [DOI] [PubMed] [Google Scholar]
- Cui M., Chen J., Myers T. R., Hwang B. J., Sternberg P. W., et al. , 2006. SynMuv genes redundantly inhibit lin-3/EGF expression to prevent inappropriate vulval induction in C. elegans. Dev. Cell 10: 667–672. [DOI] [PubMed] [Google Scholar]
- Dittmer J., 2003. The biology of the Ets1 proto-oncogene. Mol. Cancer 2: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantz D. A., Jacobs D., Glossip D., Kornfeld K., 2001. Docking sites on substrate proteins direct extracellular signal-regulated kinase to phosphorylate specific residues. J. Biol. Chem. 276: 27256–27265. [DOI] [PubMed] [Google Scholar]
- Farooqui S., Pellegrino M. W., Rimann I., Morf M. K., Muller L., et al. , 2012. Coordinated lumen contraction and expansion during vulval tube morphogenesis in Caenorhabditis elegans. Dev. Cell 23: 494–506. [DOI] [PubMed] [Google Scholar]
- Feilotter H. E., Hannon G. J., Ruddell C. J., Beach D., 1994. Construction of an improved host strain for two hybrid screening. Nucleic Acids Res. 22: 1502–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E. L., Horvitz H. R., 1989. The multivulva phenotype of certain Caenorhabditis elegans mutants results from defects in two functionally redundant pathways. Genetics 123: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E. L., Sternberg P. W., Horvitz H. R., 1987. A genetic pathway for the specification of the vulval cell lineages of Caenorhabditis elegans. Nature 326: 259–267. [DOI] [PubMed] [Google Scholar]
- Foulds C. E., Nelson M. L., Blaszczak A. G., Graves B. J., 2004. Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Mol. Cell. Biol. 24: 10954–10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioni J. V., Neel B. G., 1993. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal. Biochem. 210: 179–187. [DOI] [PubMed] [Google Scholar]
- Gill G., 2003. Post-translational modification by the small ubiquitin-related modifier SUMO has big effects on transcription factor activity. Curr. Opin. Genet. Dev. 13: 108–113. [DOI] [PubMed] [Google Scholar]
- Girdwood D., Bumpass D., Vaughan O. A., Thain A., Anderson L. A., et al. , 2003. P300 transcriptional repression is mediated by SUMO modification. Mol. Cell 11: 1043–1054. [DOI] [PubMed] [Google Scholar]
- Greenwald I. S., 1997. Development of the vulva, pp. 519–541 in C. elegans II, edited by Riddle D. L., Blumenthal T., Meyer B. J., Preiss J. R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed] [Google Scholar]
- Guerry F., Marti C., Zhang Y., Moroni P. S., Jaquiery E., et al. , 2007. The Mi-2 nucleosome-remodeling protein LET-418 is targeted via LIN-1/ETS to the promoter of lin-39/Hox during vulval development in C. elegans. Dev. Biol. 306: 469–479. [DOI] [PubMed] [Google Scholar]
- Han M., Sternberg P. W., 1990. let-60, a gene that specifies cell fates during C. elegans vulval induction, encodes a ras protein. Cell 63: 921–931. [DOI] [PubMed] [Google Scholar]
- Han M., Golden A., Han Y., Sternberg P. W., 1993. C. elegans lin-45 raf gene participates in let-60 ras-stimulated vulval differentiation. Nature 363: 133–140. [DOI] [PubMed] [Google Scholar]
- Hay R. T., 2005. SUMO: a history of modification. Mol. Cell 18: 1–12. [DOI] [PubMed] [Google Scholar]
- Herman M. A., Ch’ng Q., Hettenbach S. M., Ratliff T. M., Kenyon C., et al. , 1999. EGL-27 is similar to a metastasis-associated factor and controls cell polarity and cell migration in C. elegans. Development 126: 1055–1064. [DOI] [PubMed] [Google Scholar]
- Herman R. K., Hedgecock E. M., 1990. Limitation of the size of the vulval primordium of Caenorhabditis elegans by lin-15 expression in surrounding hypodermis. Nature 348: 169–171. [DOI] [PubMed] [Google Scholar]
- Hill R. J., Sternberg P. W., 1992. The gene lin-3 encodes an inductive signal for vulval development in C. elegans. Nature 358: 470–476. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R., Sternberg P. W., 1991. Multiple intercellular signalling systems control the development of the Caenorhabditis elegans vulva. Nature 351: 535–541. [DOI] [PubMed] [Google Scholar]
- Howard R. M., Sundaram M. V., 2002. C. elegans EOR-1/PLZF and EOR-2 positively regulate Ras and Wnt signaling and function redundantly with LIN-25 and the SUR-2 Mediator component. Genes Dev. 16: 1815–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu V., Zobel C. L., Lambie E. J., Schedl T., Kornfeld K., 2002. Caenorhabditis elegans lin-45 raf is essential for larval viability, fertility and the induction of vulval cell fates. Genetics 160: 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D., Beitel G. J., Clark S. G., Horvitz H. R., Kornfeld K., 1998. Gain-of-function mutations in the Caenorhabditis elegans lin-1 ETS gene identify a C-terminal regulatory domain phosphorylated by ERK MAP kinase. Genetics 149: 1809–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D., Glossip D., Xing H., Muslin A. J., Kornfeld K., 1999. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 13: 163–175. [PMC free article] [PubMed] [Google Scholar]
- Janknecht R., Ernst W. H., Pingoud V., Nordheim A., 1993. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 12: 5097–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.-S., Wu Y.-C., 2014. LIN-3/EGF promotes the programmed cell death of specific cells in Caenorhabditis elegans by transcriptional activation of the pro-apoptotic gene egl-1. PLoS Genet. 10: e1004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., et al. , 2003. Systemic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. [DOI] [PubMed] [Google Scholar]
- Kornfeld K., 1997. Vulval development in Caenorhabditis elegans. Trends Genet. 13: 55–61. [DOI] [PubMed] [Google Scholar]
- Kornfeld K., Guan K. L., Horvitz H. R., 1995. The Caenorhabditis elegans gene mek-2 is required for vulval induction and encodes a protein similar to the protein kinase MEK. Genes Dev. 9: 756–768. [DOI] [PubMed] [Google Scholar]
- Lachner M., O’Carroll D., Rea S., Mechtler K., Jenuwein T., 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410: 116–120. [DOI] [PubMed] [Google Scholar]
- Lackner M. R., Kornfeld K., Miller L. M., Horvitz H. R., Kim S. K., 1994. A MAP kinase homolog, mpk-1, is involved in ras-mediated induction of vulval cell fates in Caenorhabditis elegans. Genes Dev. 8: 160–173. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Ohmachi M., Arur S., Nayak S., Francis R., et al. , 2007. Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics 177: 2039–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leight E. R., Glossip D., Kornfeld K., 2005. Sumoylation of LIN-1 promotes transcriptional repression and inhibition of vulval cell fates. Development 132: 1047–1056. [DOI] [PubMed] [Google Scholar]
- Lu X., Horvitz H. R., 1998. lin-35 and lin-53, two genes that antagonize a C. elegans Ras pathway, encode proteins similar to Rb and its binding protein RbAp48. Cell 95: 981–991. [DOI] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R., 1993. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell 73: 381–393. [DOI] [PubMed] [Google Scholar]
- Miley G. R., Fantz D., Glossip D., Lu X., Saito R. M., et al. , 2004. Identification of residues of the Caenorhabditis elegans LIN-1 ETS domain that are necessary for DNA binding and regulation of vulval cell fates. Genetics 167: 1697–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S. J., Schneider R., Bauer U. M., Bannister A. J., Morrison A., et al. , 2001. Rb targets histone H3 methylation and HP1 to promoters. Nature 412: 561–565. [DOI] [PubMed] [Google Scholar]
- O’Neill E. M., Rebay I., Tjian R., Rubin G. M., 1994. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell 78: 137–147. [DOI] [PubMed] [Google Scholar]
- Piano F., Schetter A. J., Morton D. G., Gunsalus K. C., Reinke V., et al. , 2002. Gene clustering based on RNAi phenotypes of ovary-enriched genes in C. elegans. Curr. Biol. 12: 1959–1964. [DOI] [PubMed] [Google Scholar]
- Poulin G., Dong Y., Fraser A. G., Hopper N. A., Ahringer J., 2005. Chromatin regulation and sumoylation in the inhibition of Ras-induced vulval development in Caenorhabditis elegans. EMBO J. 24: 2613–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebay I., Rubin G. M., 1995. Yan functions as a general inhibitor of differentiation and is negatively regulated by activation of the Ras1/MAPK pathway. Cell 81: 857–866. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T., 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sampson D. A., Wang M., Matunis M. J., 2001. The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J. Biol. Chem. 276: 21664–21669. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D., 1989. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16: 339–346. [DOI] [PubMed] [Google Scholar]
- Singh N., Han M., 1995. sur-2, a novel gene, functions late in the let-60 ras-mediated signaling pathway during Caenorhabditis elegans vulval induction. Genes Dev. 9: 2251–2265. [DOI] [PubMed] [Google Scholar]
- Solari F., Ahringer J., 2000. NURD-complex genes antagonise Ras-induced vulval development in Caenorhabditis elegans. Curr. Biol. 10: 223–226. [DOI] [PubMed] [Google Scholar]
- Solari F., Bateman A., Ahringer J., 1999. The Caenorhabditis elegans genes egl-27 and egr-1 are similar to MTA1, a member of a chromatin regulatory complex, and are redundantly required for embryonic patterning. Development 126: 2483–2494. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B., Koski L. B., Walsh A., Marschall P., Neumann B., et al. , 2005. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature 434: 462–469. [DOI] [PubMed] [Google Scholar]
- Sternberg, P.W., 2005 Vulval development (June 25, 2005), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.6.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. L., Cantin G. T., Wang G., Shevchenko A., Shevchenko A., et al. , 2002. Transcription control by E1A and MAP kinase pathway via Sur2 mediator subunit. Science 296: 755–758. [DOI] [PubMed] [Google Scholar]
- Stielow B., Sapetschnig A., Kruger I., Kunert N., Brehm A., et al. , 2007. Identification of SUMO-dependent chromatin-associated transcriptional repression components by a genome-wide RNAi screen. Mol. Cell 29: 742–754. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Horvitz H. R., 1981. Abnormal cell lineages in mutants of the nematode Caenorhabditis elegans. Dev. Biol. 82: 41–55. [DOI] [PubMed] [Google Scholar]
- Sundaram M. V., 2013 Canonical RTK-Ras-ERK signaling and related alternative pathways. WormBook. 11: 1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. B., Lackner M. R., Kim S. K., 1998. MAP kinase signaling specificity mediated by the LIN -1 Ets/LIN-31 WH transcription factor complex during C. elegans vulval induction. Cell 93: 569–580. [DOI] [PubMed] [Google Scholar]
- Tiensuu T., Larsen M. K., Vernersson E., Tuck S., 2005. lin-1 has both positive and negative functions in specifying multiple cell fates induced by Ras/MAP kinase signaling in C. elegans. Dev. Biol. 286: 338–351. [DOI] [PubMed] [Google Scholar]
- Timmons L., Fire A., 1998. Specific interference by ingested dsRNA. Nature 395: 854. [DOI] [PubMed] [Google Scholar]
- Tong J. K., Hassig C. A., Schnitzler G. R., Kingston R. E., Schreiber S. L., 1998. Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature 395: 917–921. [DOI] [PubMed] [Google Scholar]
- Treisman R., 1994. Ternary complex factors: growth factor regulated transcriptional activators. Curr. Opin. Genet. Dev. 4: 96–101. [DOI] [PubMed] [Google Scholar]
- Trent C., Tsung N., Horvitz H. R., 1983. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104: 619–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unhavaithaya Y., Shin T. H., Miliaras N., Lee J., Oyama T., et al. , 2002. MEP-1 and a homolog of the NURD complex component Mi-2 act together to maintain germline-soma distinctions in C. elegans. Cell 111: 991–1002. [DOI] [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A., 1993. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell 74: 205–214. [DOI] [PubMed] [Google Scholar]
- von Zelewsky T., Palladino F., Brunschwig K., Tobler H., Hajnal A., et al. , 2000. The C. elegans Mi-2 chromatin-remodelling proteins function in vulval cell fate determination. Development 127: 5277–5284. [DOI] [PubMed] [Google Scholar]
- Wade P. A., Jones P. L., Vermaak D., Wolffe A. P., 1998. A multiple subunit Mi-2 histone deacetylase from Xenopus laevis cofractionates with an associated Snf2 superfamily ATPase. Curr. Biol. 8: 843–846. [DOI] [PubMed] [Google Scholar]
- Wagmaister J. A., Miley G. R., Morris C. A., Gleason J. E., Miller L. M., et al. , 2006. Identification of cis-regulatory elements from the C. elegans Hox gene lin-39 required for embryonic expression and for regulation by the transcription factors LIN-1, LIN-31 and LIN-39. Dev. Biol. 297: 550–565. [DOI] [PubMed] [Google Scholar]
- Wang H. B., Zhang Y., 2001. Mi2, an auto-antigen for dermatomyositis, is an ATP-dependent nucleosome remodeling factor. Nucleic Acids Res. 29: 2517–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Han M., 1994. Suppression of activated Let-60 ras protein defines a role of Caenorhabditis elegans Sur-1 MAP kinase in vulval differentiation. Genes Dev. 8: 147–159. [DOI] [PubMed] [Google Scholar]
- Wu Y., Han M., Guan K. L., 1995. MEK-2, a Caenorhabditis elegans MAP kinase kinase, functions in Ras-mediated vulval induction and other developmental events. Genes Dev. 9: 742–755. [DOI] [PubMed] [Google Scholar]
- Xue Y., Wong J., Moreno G. T., Young M. K., Cote J., et al. , 1998. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol. Cell 2: 851–861. [DOI] [PubMed] [Google Scholar]
- Yang S. H., Sharrocks A. D., 2004. SUMO promotes HDAC-mediated transcriptional repression. Mol. Cell 13: 611–617. [DOI] [PubMed] [Google Scholar]
- Yang S. H., Bumpass D. C., Perkins N. D., Sharrocks A. D., 2002. The ETS domain transcription factor Elk-1 contains a novel class of repression domain. Mol. Cell. Biol. 22: 5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. H., Jaffray E., Hay R. T., Sharrocks A. D., 2003. Dynamic interplay of the SUMO and ERK pathways in regulating Elk-1 transcriptional activity. Mol. Cell 12: 63–74. [DOI] [PubMed] [Google Scholar]
- Zhang X., Greenwald I., 2011. Spatial regulation of lag-2 transcription during vulval precursor cell fate patterning in Caenorhabditis elegans. Genetics 188: 847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., LeRoy G., Seelig H. P., Lane W. S., Reinberg D., 1998. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell 95: 279–289. [DOI] [PubMed] [Google Scholar]