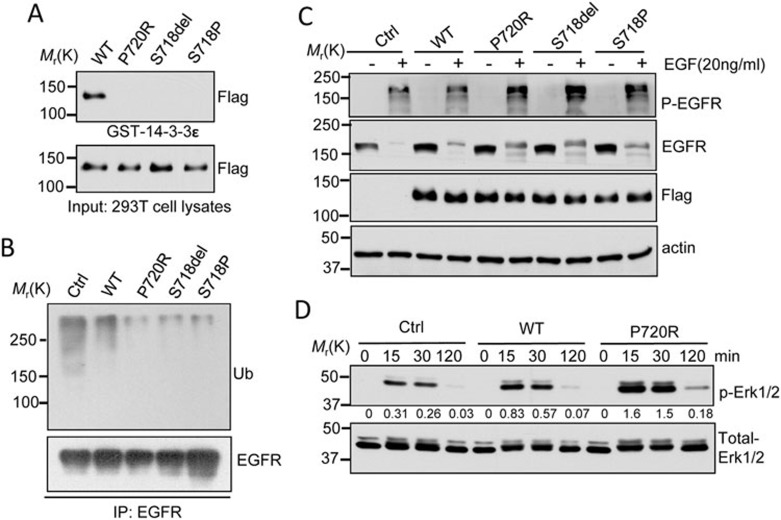
Figure 2.
Molecular characterization of USP8 mutants. (A) USP8 mutants fail to bind 14-3-3 protein. Cell lysates from 293T cells expressing Flag-tagged WT-USP8 or the indicated USP8 mutants were incubated with GST-14-3-3ε immobilized on Glutathione-Sepharose beads. GST pull-down or input samples were immunoblotted with anti-Flag antibody. (B) Reduced EGFR ubiquitination in USP8 mutants. Cell lysates from HeLa cells stably expressing Flag-tagged WT-USP8, the indicated mutant or the vector only (Ctrl) were immunoprecipitated (IP) using anti-EGFR antibody, and the immunoprecipitation products were analyzed by immunoblotting with anti-EGFR and ubiquitin (Ub) antibodies. (C) Slower EGFR degradation in HeLa cells expressing USP8 mutant relative to WT. Serum-starved HeLa cells stably expressing Flag-tagged USP8 protein were treated with EGF (20 ng/ml) in the presence of cycloheximide (25 μg/ml) for 3 h. Immunoblot analysis was performed to determine p-EGFR, EGFR and Flag-USP8 protein levels. (D) Enhanced EGF-induced Erk phosphorylation in USP8 P720R mutant. HeLa cells stably expressing WT, USP8 P720R mutants or the control were treated with EGF (20 ng/ml) for the indicated times. Cell lysates were subjected to immunoblot analysis for total Erk and phospho-Erk (p-Erk). The densitometric ratio of p-Erk1/2 to total-Erk1/2 is shown between panels. Three independent experiments were repeated with similar results.