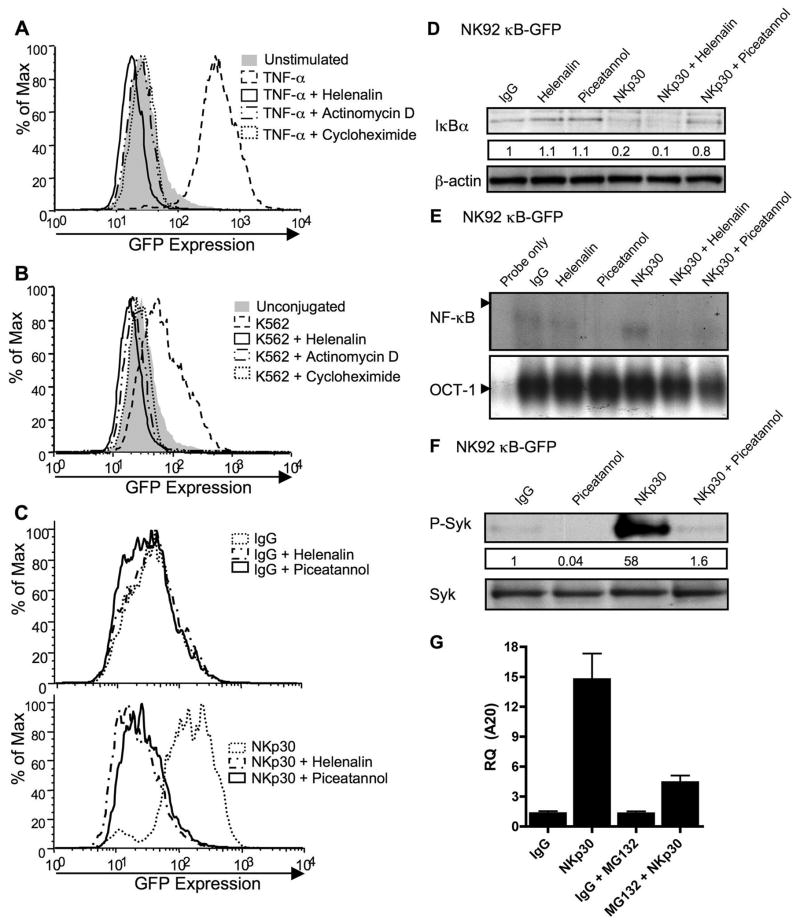
Figure 9. Activation-induced NF-κB function in NK92 cells.
NK92 cells transduced with a κB-GFP reporter construct were stimulated with TNF-α (A), K562 target cells (B), or immobilized IgG (top) or anti-NKp30 (bottom) (C) for 8h after 30 min pretreatment with media, helenalin, actinomycin-D, cycloheximide or piceatannol. GFP expression in live cells was analyzed by FACS and representative results of five independent experiments are shown. NK92 κB-GFP cells were pretreated for 30min with media, helenalin, or piceatannol and stimulated for 30min with immobilized IgG or anti-NKp30. Whole cell lysates were evaluated for the presence of IκBα by Western blot (D) and nuclear extracts for NF-κB (top) and OCT-1 (bottom) DNA binding activity by EMSA (E). Whole cell lysates of NK92 κB-GFB cells pretreated for 30 min with media or piceatannol and stimulated for 30 min with IgG or NKp30 were evaluated by Western blot for phospho-Syk (F, top). The membrane was stripped and reprobed for total Syk (F, bottom). The numbers below the phospho-Syk blot represent the ratio of the phospho-Syk band intensity to the IgG phospho-Syk band. (G) A20 transcripts were quantified using real-time PCR from NK92-κB GFP cells that were pretreated for 30 min with media, or MG132 and then stimulated for 4 h with immobilized IgG or anti-NKp30.