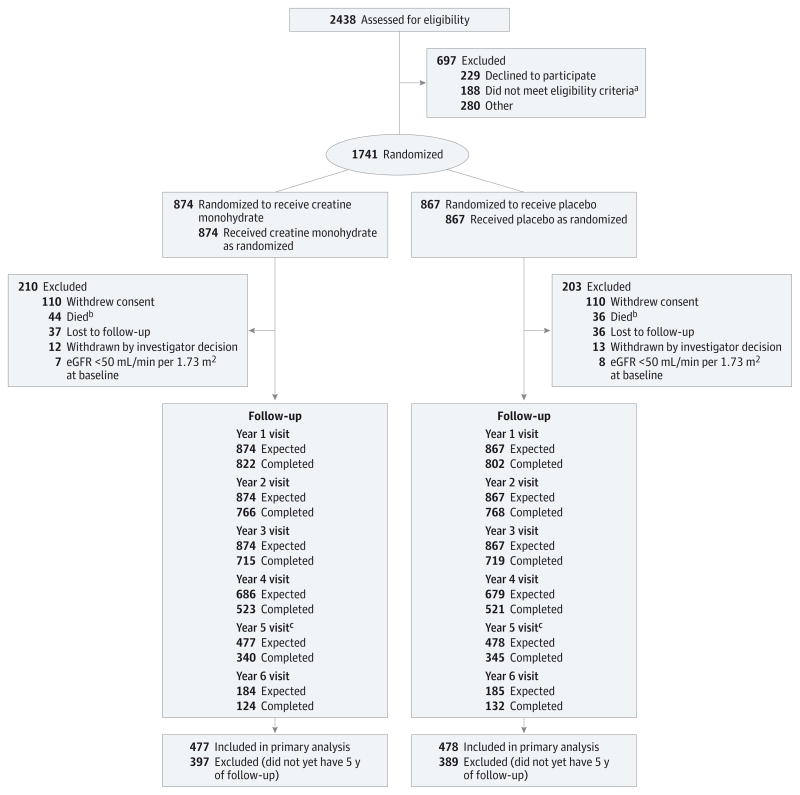
Figure. CONSORT Diagram of Long-term Study 1 Trial Enrollment Status From Initiation of Screening to the Time of the Interim Analysis on July 17, 2013.
aTaking exclusionary medications (n = 67), not taking dopaminergic therapy (n = 34), diagnosis uncertain (n = 24), medical condition (n = 22), Parkinson disease too advanced (n = 22), enrolled in another study (n = 4), inability to consent (n = 2), non–English-speaking (n = 2), or failure to meet other inclusion criteria (n = 11).
bNine additional deaths occurred after participants withdrew consent (creatine monohydrate, n = 4; placebo, n = 5).
cCohort (cohort 1) used in the interim efficacy analyses.