Abstract
Background/Objectives
To determine whether body composition measures differ between metabolically healthy obese (MHO) and metabolically abnormal obese (OA) adults.
Subjects/Methods
The sample included 395 obese adults from the Pennington Center Longitudinal Study, 18–68 years of age. Adults were classified as OA (≥2 cardiometabolic risk factors: blood pressure ≥130/85 mmHg; triglycerides ≥150 mg/dL, high density lipoprotein cholesterol: men <40, women <50 mg/dL; fasting glucose ≥100 mg/dL) or MHO (<2 cardiometabolic risk factors). Whole-body bone mineral density (BMD; g/cm2), bone mineral content (BMC; kg), percent body fat (%), fat mass (kg), lean mass (kg) and trunk adipose tissue mass (kg) were measured with dual-energy x-ray absorptiometry. Visceral (VAT; cm2), subcutaneous (SAT; cm2), and total abdominal adipose tissue (TAT; cm2) were measured with computed tomography. Gender-specific general linear regression models were used to determine differences in body composition between MHO and OA controlling for age, race, smoking status, and menopause status (in women).
Results
In men, OA had greater fat mass (OA vs. MHO mean ± SE; p-value for difference: 31.4±1.2 vs. 28.6±1.2 kg; p=0.02) and greater trunk adipose tissue (16.5±0.7 vs. 14.3±0.8 kg; p=0.002) compared with MHO, but no significant differences between MHO and OA profiles for BMD, BMC, % fat, lean mass, VAT, SAT, or TAT. Women with OA profiles had greater lean mass (54.4±1.0 vs. 51.5±1.0 kg; p<0.0001), greater VAT (119.4±1.1 vs. 95.7±1.1 cm2; p<0.0001) and greater trunk adipose tissue (18.0±0.5 vs. 17.1±0.5 kg; p=0.03) when compared with MHO women, with no significant differences between MHO and OA for BMD, BMC, % fat, fat mass, SAT or TAT.
Conclusion
OA and MHO cardiometabolic profiles are characterized by differences in body composition that vary by gender. Men have differences in overall and trunk adipose tissue while women have differences in lean mass and centralized fat (VAT and trunk).
Keywords: VAT, SAT, adipose tissue, DXA, CT
Introduction
Obesity has been recognized as a complex and heterogeneous condition, whereby despite the presence of excess adipose tissue, varying cardiometabolic risk may occur. Consequently, varying phenotypes exist within obesity, and have been identified as metabolically healthy obese (MHO) and metabolically abnormal obese (OA). Those with the MHO phenotype have favorable levels of cardiometabolic risk factors (1–3), lower risk for cardiovascular disease (4), diabetes (4), and mortality (5) when compared with their OA counterparts.
Comparisons of body composition between MHO and OA profiles have been limited to mostly women (1, 6–10) and/or non-U.S.- based adults (6, 9, 11, 12). The few U.S. based studies have had combined statistical analyses for men and women (13), who have known differences in adipose tissue storage (14, 15). Previous studies have also mostly focused on white/Caucasian populations (11–13), despite body composition differences between adults of varying race/ethnicities (14). Further, we know that body composition changes with aging (14, 16), and many of the aforementioned studies have focused on older postmenopausal women (1, 2, 7, 8, 10).
Thus, a major limitation in the current literature has been a lack of focus on possible differences in regional and total adipose tissue, taking into account gender, age, and race/ethnicity. Therefore, the purpose of this study is to determine whether measures of body composition differ between MHO and OA using a U.S. based sample of men and women.
Subjects and Methods
The sample included 395 obese (BMI ≥30 kg/m2) men and women (≥ 18 years of age) from the Pennington Center Longitudinal Study (PCLS). PCLS is made up of volunteers visiting the Pennington Biomedical Research Center (PBRC) in Baton Rouge, Louisiana, who have participated in nutrition, weight loss and other metabolic observational and intervention studies since 1992 (17). The current cross-sectional study is limited to participants who were screened at PBRC (between 1996 and 2010) and who had baseline dual-energy x-ray absorptiometry (DXA) whole body scans, computed tomography abdominal (CT) scans, and blood draws for cardiovascular risk factors. Participants were excluded if pregnant. Each volunteer provided their written informed consent. All PCLS procedures and secondary analyses were approved by the PBRC Institutional Review Board.
Whole-body bone mineral density (BMD; g/cm2), bone mineral content (BMC; kg), percent body fat (%), fat mass (kg), lean mass (kg) and trunk and appendage adipose tissue mass (kg) were measured with DXA. Two Hologic models (Bedford, MA) were utilized for imaging: the QDR2000 (n=387) was phased out in 2006, and replaced with the QDR4500 (n=415) which has been in service since 2001. Concordance between the two DXA machines was determined with same-day scans on a subsample of participants (n=32), resulting in a high degree of agreement for percent fat measurements (R2= 0.987), FM (R2= 0.993), lean mass (R2= 0.957), and BMD (R2= 0.901). Equations were used to convert the QDR2000 data to QDR4500 data for percent fat (Y=0.8015x + 2.3903), fat mass (Y=0.8323x + 1527.7), lean mass (Y=1.016x + 4511.8), trunk fat (Y=0.824x+1121.7), BMC (Y=0.8151x+216.16), and BMD (Y=0.8712x + 0.1013). In order to assess reliability, coefficients of variation (CV; mean ± SD) were also calculated for repeated DXA measures obtained 14 days apart on a subsample (n=88) for BMD (0.8 ± 0.5 %), lean mass (1.2 ±0.8 %) and fat mass (1.8 ± 1.4 %). Each DXA used a phantom prior to data collection for calibration and to document stability of measures over time. Manufacturer calibrations were performed twice a year as recommended. Each participant’s scan was analyzed with the latest software QDR for Windows V11.2.
Visceral adipose tissue (VAT; cm2), subcutaneous adipose tissue (SAT; cm2), and total abdominal adipose tissue (TAT; cm2) were measured with CT. Abdominal VAT and SAT procedures for measuring cross sectional area (cm2) have been previously described(18). Briefly, CT scans occurred at the Baton Rouge General Medical Center, Baton Rouge, Louisiana with 3 different CT scanners: GE High Speed Advantage; GE LightSpeed Plus; and GE LightSpeed VCT (all GE Healthcare, Princeton, NJ, USA). CT scanners were calibrated daily to air, and to obtain a cross-sectional image at the L4-L5 intervertebral space, participants lay supine with arms overhead. CT imaging was analyzed at PBRC with commercially available software (Analyze; Analyze Direct, Rochester, MN).
Participants were asked to not engage in vigorous exercise, ingest food or caffeine or smoke within 30 min of blood pressure measurement. All blood pressure measurements were taken manually using the appropriate cuff size, a stethoscope and standard sphygmomanometer, or in some cases using a validated Omron automatic measuring device (Omron, Bannockburn, IL,USA). Resting BP measurements were obtained after a 5-min rest, with the participant in a semi-recumbent position in a quiet room. The Korotkoff sounds were used to establish the first-and fifth phases. Each measurement was taken twice, 1–2 minutes apart, and the average of the two measurements was recorded in mmHg.
Blood was collected following a minimum of a 10 hour fast, and within 60 days of the body scans. Glucose and lipids were analyzed on a Beckman Coulter DXC600 (Beckman Coulter, Brea, CA, USA). Adults were classified as OA (≥2 cardiometabolic risk factors: blood pressure ≥130/85 mmHg; triglycerides ≥150 mg/dL, high density lipoprotein cholesterol (HDL-C) men <40, women <50 mg/dL; fasting glucose ≥100 mg/dL) or MHO (0 or 1 cardiometabolic risk factor).
Participant age was computed from birth and observation dates. Sex, race/ethnicity and smoking status were self-reported by questionnaire during the recruitment process. Participants were classified as “non-smokers”, “current smokers” or “former smokers”. Menopausal status (pre-menopausal/post-menopausal) was determined in women from their age and responses to questions regarding their reproductive history. Women ≥ 55 years of age, or those who indicated that they can no longer have children because of achieving menopause were considered to be post-menopausal.
Statistical Analysis
All analyses were performed with SAS 9.3, and statistical significance was defined as p < 0.05. Differences between MHO and OA were explored with t-tests between the unadjusted demographic characteristics, cardiovascular risk factors, and body composition parameters within gender groups. Non-normally distributed variables were log transformed for analysis (lean mass, VAT, BMD and BMC) but means were reverse-transformed for presentation of results. Gender-specific general linear regression models were used to determine differences in body composition between MHO and OA controlling for age, race, smoking status, and menopausal status (in women).
Results
The PCLS sample was composed of 66% women; 38% African American, and ranged in age from 18–68 years of age (mean ± SD: 40.6 ± 13.2 years). MHO adults were significantly younger age in both men and women, with a lower weight, BMI and waist circumference compared with OA (Table 1). In both men and women with the MHO profile, systolic blood pressure, diastolic blood pressure, glucose, and triglycerides were all significantly lower, while HDL-C was significantly higher when compared with OA.
Table 1.
Demographic characteristics and cardiometabolic risk factors in adults from the Pennington Center Longitudinal Study (n=395).+
| Men (n=136; 34%) | Women (n=259; 66%) | ||||
|---|---|---|---|---|---|
| Total | MHO | OA | MHO | OA | |
| n (%) | 395 | (n=57; 42%) | (n=79; 58%) | (n=153; 59%) | (n=106; 41%) |
| Age (years) | 41.1 ± 11.2 | 37.8 ± 12.7* | 43.6 ± 11.2 | 39.2 ± 10.6* | 43.5 ± 10.1 |
| Race/Ethnicity | |||||
| Caucasian | 246 (62) | 47 (82) | 64 (81) | 76 (50) | 59 (57) |
| African American | 149 (38) | 10 (18) | 15 (19) | 77 (50) | 47 (44) |
| Weight (kg) | 96.2 ± 12.2 | 102.7 ± 9.6* | 107.8 ±11.2 | 89.8 ± 8.9* | 93.2 ± 10.6 |
| Height (cm) | 168.3 ± 8.8 | 176.4 ± 6.0 | 178.1 ± 5.7 | 163.4 ± 6.1 | 163.6 ± 5.4 |
| BMI (kg/m2) | 33.9 ± 3.0 | 33.0 ± 2.1* | 34.0 ± 3.2 | 33.6 ± 2.8* | 34.8 ± 3.4 |
| WC (cm) | 102.3 ± 10.9 | 107.1 ± 7.9* | 111.8 ± 11.5 | 96.4 ± 8. * | 101.3 ± 8.8 |
| Smoking Status n (%) | |||||
| Never | 301 (76) | 40 (70) | 48 (61) | 131 (86) | 82 (77) |
| Former | 75 (19) | 14 (25) | 28 (35) | 17 (11) | 16 (15) |
| Current | 19 (5) | 3 (5) | 3 (4) | 5 (3) | 8 (8) |
| Post-menopausal (%) | N/A | N/A | N/A | 16 (10)* | 21 (20) |
| Systolic BP (mmHg) | 120.5 ± 12.9 | 118.8 ± 11.2* | 127.2 ± 11.5 | 116.0 ± 12.0* | 122.9 ± 13.4 |
| Diastolic BP (mmHg) | 77.8 ± 8.4 | 75.5 ± 7.2* | 83.1 ± 7.2 | 75.1 ± 7.7* | 79.2 ± 8.8 |
| Glucose (mg/dL) | 98.4 ± 13.2 | 95.4 ± 4.7* | 106.3 ±21.8 | 93.2 ± 7.8* | 101.6 ± 9.8 |
| Triglycerides (mg/dL) | 131.7 ± 73.1 | 122.2 ± 57.1* | 182.3 ± 89.6 | 89.8 ± 41.4* | 159.7 ± 67.4 |
| HDL Cholesterol (mg/dL) | 49.8 ± 12.3 | 44.6 ± 8.1* | 40.9 ± 9.5 | 58.9 ± 11.5* | 46.2 ± 8.3 |
Data presented as mean ± standard deviation
Significant difference in the unadjusted means between OA and MHO within gender
In the unadjusted body composition analyses comparing MHO and OA, men with MHO had significantly lower amounts of fat mass (kg), trunk fat (kg), VAT, and TAT when compared to OA (Table 2). Women with MHO had significantly lower lean mass (kg), VAT and trunk fat (kg) in comparison with OA (Table 2). No other body composition variables were significantly different between MHO and OA phenotypes in the unadjusted analyses.
Table 2.
Unadjusted body composition values from DEXA scans in the PCLS between MHO and OA phenotypes.
| Men | Women | |||
|---|---|---|---|---|
| MHO | OA | MHO | OA | |
| DEXA | ||||
| BMD (g/cm2) | 1.24 ± 0.10 | 1.21 ± 0.12 | 1.12 ± 0.08 | 1.13 ± 0.10 |
| BMC (kg) | 2.92 ± 0.36 | 2.84 ±0.39 | 2.29 ± 0.28 | 2.31 ± 0.31 |
| Fat (%) | 29.0 ± 4.2 | 30.5 ± 4.5 | 41.6 ± 3.7 | 41.0 ± 3.3 |
| Fat mass (kg) | 29.7 ± 5.9* | 32.7 ±6.9 | 37.6 ± 5.9 | 38.4 ± 6.0 |
| Lean mass (kg) | 72.7 ± 7.4 | 74.4 ± 7.7 | 51.4 ± 5.2* | 53.9 ± 6.1 |
| Appendage Adipose Tissue (kg) | 13.3 ± 2.9 | 13.5 ±3.1 | 19.4 ± 3.8 | 19.0 ± 3.7 |
| Trunk Adipose Tissue (kg) | 15.2 ± 3.9* | 17.9 ± 4.5 | 17.3 ±3.3* | 18.4 ± 3.5 |
| CT Scan | ||||
| VAT (cm2) | 139.0 ± 60.1* | 170.9 ± 71.6 | 108.7 ± 53.2* | 146.7 ± 61.8 |
| SAT (cm2) | 393.3 ± 99.3 | 410.5 ±108.9 | 532.2 ± 100.0 | 524.0 ± 106.7 |
| TAT (cm2) | 532.3 ± 116.9* | 581.4 ± 137.2 | 640.9 ± 117.9 | 670.7 ± 123.8 |
Significant difference between MHO and OA within gender
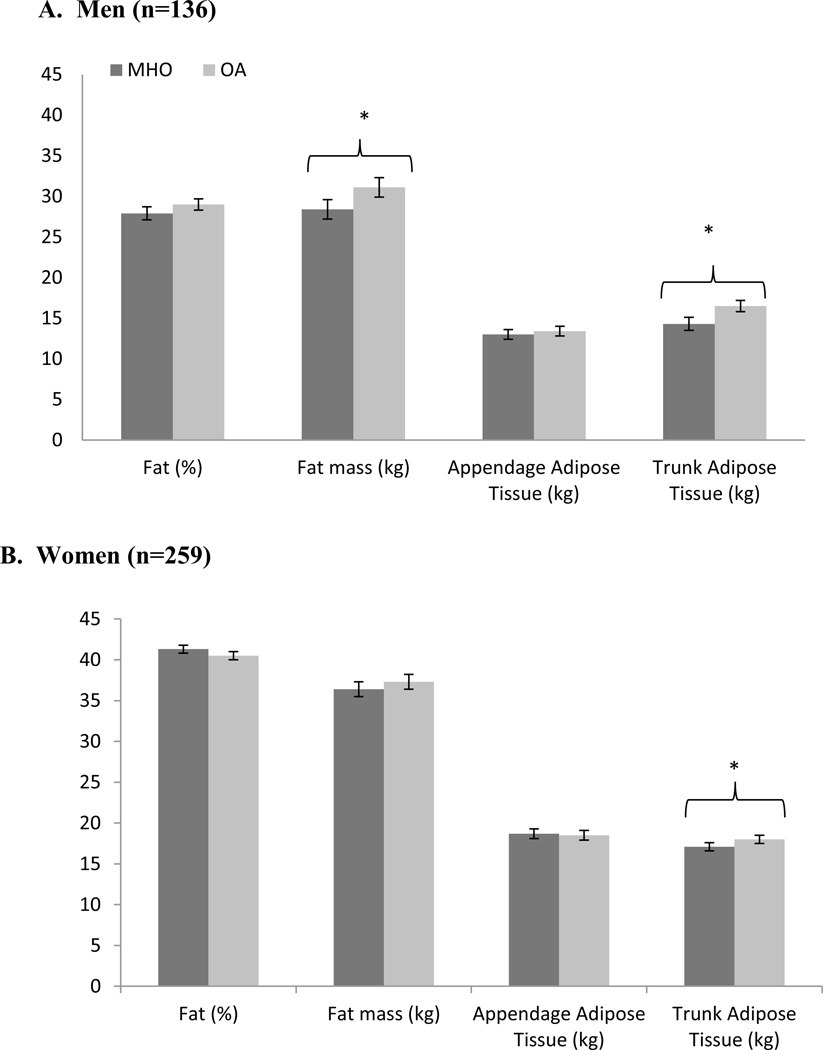
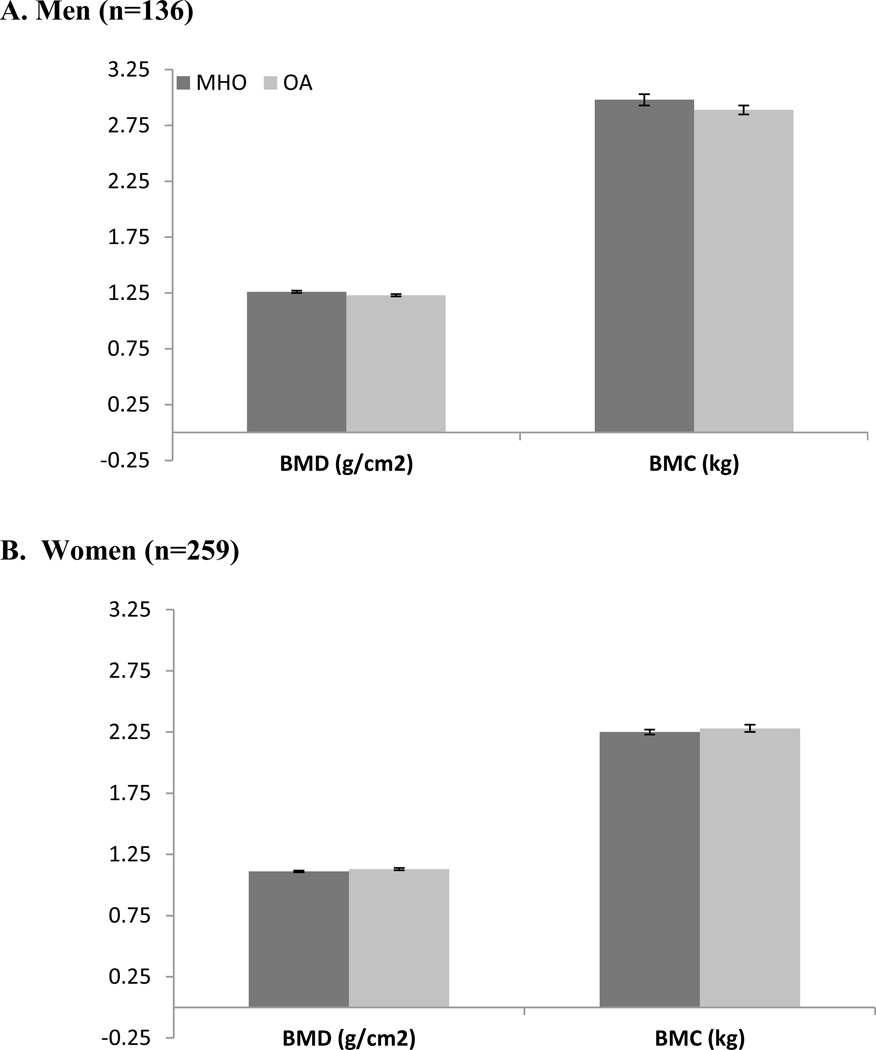
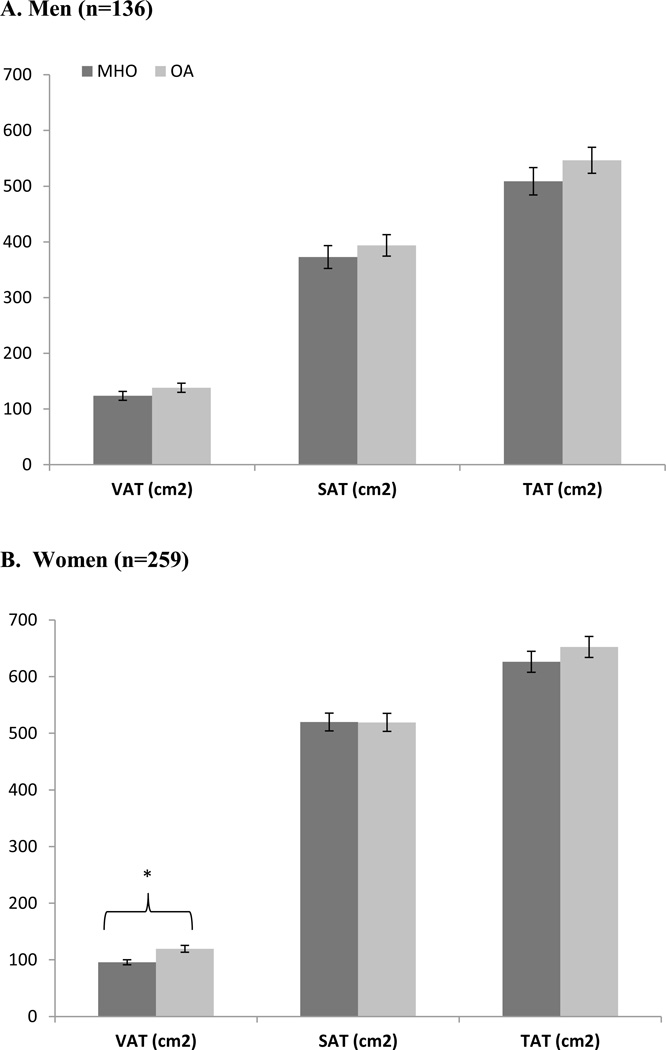
Once adjusted for age, race and smoking status, results from the DXA scan showed that men with the MHO had lower fat mass (OA vs. MHO mean ± SE; p-value for difference: 28.5 ± 1.2 vs. 31.1 ± 1.2 kg; p = 0.02) and lower trunk adipose tissue (14.3 ± 0.8 vs. 16.5 ± 0.7 vs. kg; p = 0.002) compared with OA men (Figure 1a). No significant differences were found in men between MHO and OA profiles for percent fat (Figure 1a), BMD, BMC (Figure 2a), or lean mass (Figure 3a) in the adjusted analyses. From the adjusted CT analyses, there were no significant differences between MHO and OA for VAT, SAT or TAT in men (Figure 4a).
Figure 1.
Total and regional fat mass and lean mass between MHO and OA from DXA scan in a) men and b) women in the PCLS (mean ± SE).
Figure 2.
BMD (g/cm2) and BMC (kg) comparison in MHO and OA in a) men and b) women in the PCLS (mean ± SE).
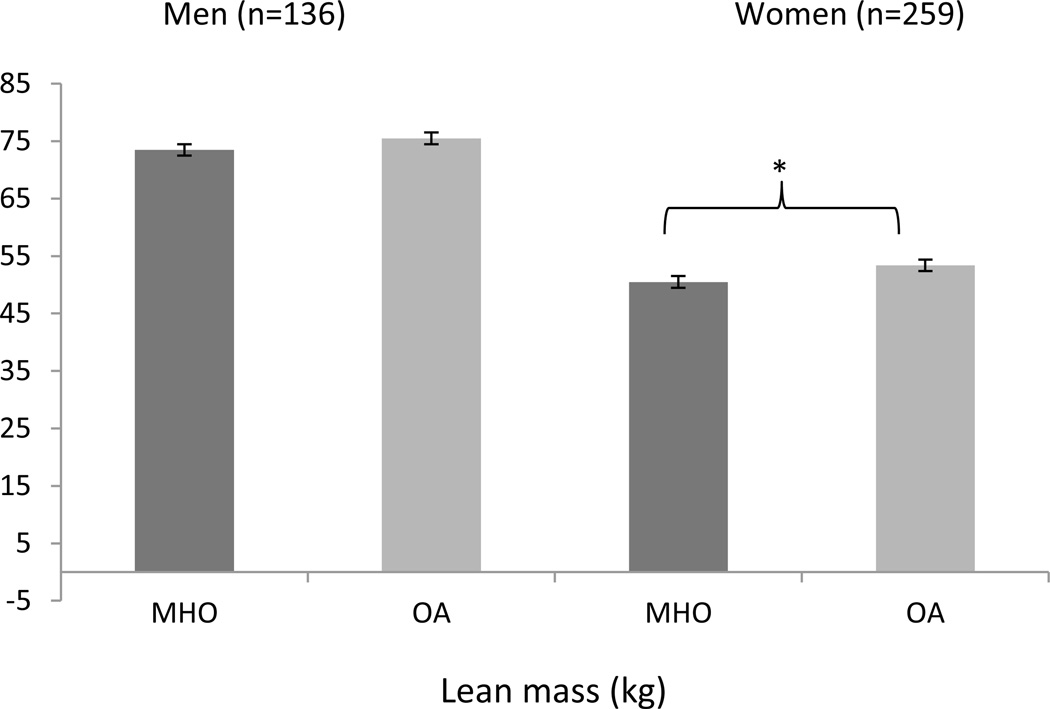
Figure 3.
Lean mass (kg) from DXA between MHO and OA men and women from the PCLS (mean ± SE).
Figure 4.
Regional fat mass from CT Scan between MHO and OA in a) men and b) women in the PCLS (mean ± SE).
In the adjusted DXA models, Women with MHO phenotypes had less trunk adipose tissue (17.1 ± 0.5 vs. 18.0 ± 0.5 kg; p = 0.03) (Figure 1b), and lower lean mass (50.5 ± 1.0 vs. 53.4 ± 1.0 kg; p < 0.0001) (Figure 3b), when compared with OA women. There were no significant differences in women between MHO and OA for % fat, fat mass (Figure 1b), BMD, or BMC (Figure 2b). In the CT adjusted models, women with MHO had lower VAT (95.7 ± 1.1 vs. 119.4 ± 1.1 cm2; p < 0.0001) (Figure 4b), but no significant differences for SAT or TAT when compared to their OA counterparts.
Discussion
In the current analyses, OA and MHO cardiometabolic profiles are characterized by differences in body composition that vary by gender. Men have differences in overall and centralized adipose tissue, while women have differences centralized adipose tissue and lean mass.
In women, previous findings have shown that VAT explains 22% of variance in insulin sensitivity between MHO and OA, after adjusting for age of onset of obesity (1). Lower levels of VAT have been confirmed in many other studies in women (1, 2, 6, 8–10). We also found that women with the MHO phenotype have lower VAT in comparison to their OA counterparts utilizing our cardiometabolic clustering definition. In contrast, for men, we did not find lower VAT in the MHO profile from the adjusted analysis. However, our results did show lower unadjusted VAT, and lower adjusted trunk fat in men from the DXA analyses, which suggests that there may be differences in centralized adipose tissue storage between MHO and OA phenotypes. Lack of significance of VAT in the adjusted analysis may be due to the lower number of men in this PCLS sample, which may have influenced our statistical power. Currently, we do not know of other studies which exclusively studied VAT in MHO and OA men. However, Stefan did not find significant differences in VAT between MHO and OA in a combined German men and women sample, though these analyses were not gender specific (11). Thus, the current study does suggest regional and central adipose tissue differences in both men and women between MHO and OA phenotypes.
Previous studies have confirmed our findings that for women, those with the MHO phenotype have lower lean mass (1, 8, 10, 19–21), and no significant differences for SAT (1, 9, 10, 20) or fat mass (10) when compared to the OA phenotype. Other studies that have included both men and women have shown equivocal results; some show significantly less fat mass in MHO (13), while others have not shown significant differences between the obesity phenotypes (11, 12), although none of these studies controlled for gender, or analyzed body composition separately in men and women. We believe this may be the first study to examine SAT, lean mass and fat mass exclusively in men between MHO and OA phenotypes. For men, we did find significantly less fat mass in men with MHO phenotype, and no differences in SAT or lean mass, however, these results would need to be replicated in future studies.
One of the challenges of the current literature examining body composition between MHO and OA is the absence of a standardized definition. Some definitions use cardiometabolic clustering (10, 13), insulin resistance (1, 6, 8, 11, 12), or a combination of both (7, 9). Some of these studies included waist circumference (9, 10), while others did not (7, 13). We chose to use the cardiometabolic clustering definition since these variables are often measured and interpreted in routine clinical care, and may be more relevant on a population level. We also excluded waist circumference in our current definition, as many obese individuals already have an elevated waist circumference which may overestimate the presence of the OA profile. Utilizing different definitions to identify obesity phenotypes may impact prevalence estimates (22), and research by Messier et al., suggests that comparisons and significant findings between MHO and OA are dependent based on the definition used in postmenopausal women (7). However, other research finds that no matter what definition is used, MHO have a significant decrease in risk for cardiovascular disease (4), diabetes (4), and mortality (5). Thus, recommendations should be made to establish a definition in order to directly compare and contrast studies in the current and future literature on obesity phenotypes.
A strength of the current study is that it employs a sample of U.S. men and women with a wide range of age (18–64 years), as well as inclusion of both white and African American adults. We were also able to control for known influences of adipose tissue in our analyses such as age, race, smoking status, and menopause (as appropriate). Although we were able to perform separate gender analyses, we were somewhat limited in our sample size for men, and this may have influenced our statistical power to detect significant differences. Further, the PCLS is a group of volunteers from Baton Rouge Louisiana, and may not be generalizable to the overall population. Despite these limitations, we were able to use precise measures of adiposity from DXA and CT scans to assess differences in MHO and OA phenotypes separately in men and women.
In conclusion, we found that body composition did differ between MHO and OA phenotypes, and that these differences were gender specific. Future studies should confirm these results in different race/ethnicity and age groups.
Acknowledgments
This research was supported by the Pennington Biomedical Research Center. This project was partially supported by a Nutrition Obesity Research Center (NIH 2P30DK072476) center grant from the National Institutes of Health. PTK is supported, in part, by the Louisiana Public Facilities Authority Endowed Chair in Nutrition. Special thanks to Emily Mire and Connie Murla for data management and to the many clinical scientists and staff of the Pennington Biomedical Research Center who have contributed data to the development of the Pennington Center Longitudinal Study.
Footnotes
Conflicts of Interest: SMC and PTK have no conflicts of interest to report.
REFERENCES
- 1.Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 2.Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004;30:569–572. doi: 10.1016/s1262-3636(07)70156-8. [DOI] [PubMed] [Google Scholar]
- 3.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 4.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 5.Durward CM, Hartman TJ, Nickols-Richardson SM. All-cause mortality risk of metabolically healthy obese individuals in NHANES III. J Obes. 2012;2012:460321. doi: 10.1155/2012/460321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennings CL, Lambert EV, Collins M, Joffe Y, Levitt NS, Goedecke JH. Determinants of insulin-resistant phenotypes in normal-weight and obese Black African women. Obesity (Silver Spring) 2008;16:1602–1609. doi: 10.1038/oby.2008.233. [DOI] [PubMed] [Google Scholar]
- 7.Messier V, Karelis AD, Prud'homme D, Primeau V, Brochu M, Rabasa-Lhoret R. Identifying metabolically healthy but obese individuals in sedentary postmenopausal women. Obesity (Silver Spring) 2010;18:911–917. doi: 10.1038/oby.2009.364. [DOI] [PubMed] [Google Scholar]
- 8.Messier V, Karelis AD, Robillard ME, Bellefeuille P, Brochu M, Lavoie JM, et al. Metabolically healthy but obese individuals: relationship with hepatic enzymes. Metabolism. 2010;59:20–24. doi: 10.1016/j.metabol.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes (Lond) 2006;30:1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- 10.You T, Ryan AS, Nicklas BJ. The metabolic syndrome in obese postmenopausal women: relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab. 2004;89:5517–5522. doi: 10.1210/jc.2004-0480. [DOI] [PubMed] [Google Scholar]
- 11.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 12.Succurro E, Marini MA, Frontoni S, Hribal ML, Andreozzi F, Lauro R, et al. Insulin secretion in metabolically obese, but normal weight, and in metabolically healthy but obese individuals. Obesity (Silver Spring) 2008;16:1881–1886. doi: 10.1038/oby.2008.308. [DOI] [PubMed] [Google Scholar]
- 13.Ortega FN, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, Blair SN. The intriguing metabolically healthy but obese phentoype: cardiovascular prognosis and role of fitness. European Heart Journal. 2012 doi: 10.1093/eurheartj/ehs174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demerath EW, Sun SS, Rogers N, Lee M, Reed D, Choh AC, et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity (Silver Spring) 2007;15:2984–2993. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21:415–430. doi: 10.1016/j.beem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8:339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 19.Brochu M, Mathieu ME, Karelis AD, Doucet E, Lavoie ME, Garrel D, et al. Contribution of the lean body mass to insulin resistance in postmenopausal women with visceral obesity: a Monet study. Obesity (Silver Spring) 2008;16:1085–1093. doi: 10.1038/oby.2008.23. [DOI] [PubMed] [Google Scholar]
- 20.Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud'homme D, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 21.Marini MA, Succurro E, Frontoni S, Hribal ML, Andreozzi F, Lauro R, et al. Metabolically healthy but obese women have an intermediate cardiovascular risk profile between healthy nonobese women and obese insulin-resistant women. Diabetes Care. 2007;30:2145–2147. doi: 10.2337/dc07-0419. [DOI] [PubMed] [Google Scholar]
- 22.Velho S, Paccaud F, Waeber G, Vollenweider P, Marques-Vidal P. Metabolically healthy obesity: different prevalences using different criteria. Eur J Clin Nutr. 2010;64:1043–1051. doi: 10.1038/ejcn.2010.114. [DOI] [PubMed] [Google Scholar]