Abstract
Silent ischemia is a common manifestation of coronary artery disease (CAD). Continuous ECG (cECG) monitoring is an effective tool for assessing the frequency and duration of silent ischemic episodes for patients with CAD and for risk stratifying asymptomatic patients or those after an acute coronary syndrome by identifying those at increased risk for future cardiovascular events or death. cECG also allows monitoring of the effectiveness of therapy in patients with CAD. Treatment strategies targeted toward the elimination of silent ischemia have shown that revascularization was better than medical therapy in eliminating silent ischemia, but large scale, prospective studies targeting silent ischemia as a treatment endpoint are still lacking. Future research is warranted to study the effects of newer medical agents or the selected use of revascularization in those patients with persistent silent ischemia despite current medical regiments.
Keywords: silent ischemia, continuous electrocardiography, ambulatory electrocardiography, Holter
Introduction
Since the invention of the continuous ECG monitor (cECG, AECG [ambulatory] ECG, or Holter) in 1961 by Norman J. Holter, the methodologies and applications of continuous recording of the ECG have evolved tremendously. The pioneering work of Bruce Del Mar led to the first commercially available cECG in 1962 and the methodologies have become refined to the degree that the devices now only weigh a few ounces and use solid-state memory to record up to a week's worth of continuous ECGs. The original cECGs were primarily used to detect disturbances in the cardiac rhythm, but early studies investigated the presence and significance of ST-segment depression.[1] With better fidelity of the low-frequency signal of the ST-segment, monitoring for the presence of myocardial ischemia has become routine.
cECG monitoring provides unique insight into the presence and severity of myocardial ischemia in patients with CAD and is primarily performed in two clinical settings: as outpatient evaluation of stable CAD patients and as inpatient evaluation of patients with ACS who need more prolonged recordings up to 7 days. cECG monitoring of patients with stable CAD allows for the assessment of patients in their usual environments where the patient is exposed to physical and emotional stresses common to daily life. Such insights are fundamentally different than assessments of ischemia in a supervised laboratory setting, where stress testing is routinely performed. In the context of a hospitalized patient with an ACS, continuous ECG monitoring provides a unique insight into the stability of the underlying pathophysiologic process and the adequacy of treatment strategies.
The purpose of this review is to discuss the significance of the presence of ischemia during cECG monitoring and to briefly review the methodologies to detect and quantify such ischemia.
Definitions, Mechanism, and Technical features
The vast majority of episodes of transient ST-segment depression during cECG monitoring occur in the absence of symptoms and have been termed “silent ischemia.”[2] Important work in the 1980s and 1990s suggested that silent ischemia was associated with worse cardiovascular outcomes.[3] The reason why certain ischemic episodes are silent is not clear, but several mechanisms have been proposed. These include: episodes of asymptomatic ischemia may be less “severe” than symptomatic episodes so that an “angina threshold” is not reached;[4] disorders of the peripheral autonomic nerves, such as diabetic neuropathy, may blunt the nociceptive signals of ischemia; increased beta-endorphin levels may decrease the central perception of myocardial-related pain;[5] and abnormal central processing of afferent pain messages from the heart may occur due to non-myocardial factors, including emotional or personal characteristics.[6]
cECG monitoring provides long-term ECG recording of ST-segment shifts, arrhythmic events, and heart rate variability in patients while they perform routine daily activities. There is a clinically significant, but limited, correlation that exists between the degree of ischemia during exercise testing and the duration of ischemia detected during cECG monitoring.[7] Technologic advances over the last 20 years have led to an increased acceptance of cECG as a modality that provides accurate and clinically meaningful information about silent ischemia in patients with CAD.
For a diagnosis of ischemia, ST-segment depression of at least 0.5 mV lasting for at least 60 seconds before returning to normal should be seen.[8] Other studies have defined ischemia as > 1 mV (1mm) ST-segment depression lasting > 1 minute. Reported rates of ST-depression sensitivity (62%) and specificity (61%) as detected by continuous cECG in patients with angiographically defined chest pain and known CAD are similar to those derived from exercise treadmill testing (67% and 65%).[9] Interpretation of ST-segment shift during cECG can be problematic in patients with multiple co morbidities including hypertension, renal disease, peripheral artery disease, and diabetes, as well as in patients with underlying electrocardiographic changes such as left ventricular strain or digitalis effects. Elevations in blood pressure in asymptomatic patients with hypertension, for instance, can cause ST-segment depression, thereby confounding the diagnosis of silent ischemia.[10] Silent ST-segment shifts can also occur in up to 30% of patients with end-stage renal disease and have partly been attributed to fluctuations in blood pressure and electrolytes.[10]
Changes in either myocardial oxygen demand or supply have been proposed as the mechanism for silent ischemia seen on cECG. Most studies have suggested a demand mechanism of ischemia by demonstrating an increase in heart rate prior to silent ischemic episodes.[11] Additionally, blood pressure has been shown to increase in the minutes preceding silent ischemia.[11]
Significance of cECG Ischemia
Stable CAD
With cECG monitoring, nearly one-half of patients with stable CAD are shown to have transient ST-segment depressions that likely represent ischemic events.[12] Silent ischemia has also been documented in patients who only have risk factors for CAD, but no previous evidence for an overt CAD diagnosis.[13-15]
Prognosis of CAD patients with silent ischemia
cECG detection of silent ischemia in patients with stable CAD has important prognostic information, potentially beyond the findings that are obtainable during exercise testing. In one study, 86 patients with stable CAD and abnormal exercise ECGs were followed for a mean of 12.5 months.[16] During follow-up, there were 21 major cardiovascular events, and all but 1 of these events occurred in patients with cECG evidence of ST-segment depression. The number of ST-segment depression events during cECG monitoring correlated with the exercise ECG findings. However, after multivariable adjustment, only ST-segment depression during cECG monitoring, and not ST-segment depression during exercise testing, significantly predicted worse outcomes. Similar results were seen by a different study of patients with CAD who were asymptomatic on anti-anginal therapies.[3] In this study, silent ischemia during cECG monitoring was a more powerful predictor of mortality than exercise duration, age, previous myocardial infarction, hypertension, diabetes, or smoking history.
Pharmacologic Treatment of Asymptomatic Ischemia in Stable CAD
Beta-blockers
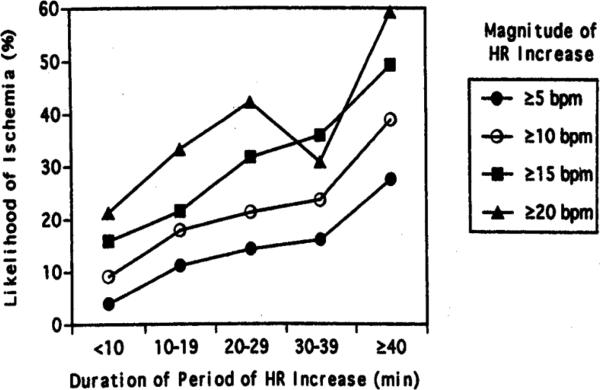
Overall, therapies that are effective in reducing myocardial oxygen demand are the most effective in suppressing daily life ischemia in patients with stable CAD. As a class, beta-adrenergic blockers are extremely effective in treating episodes of daily life ischemia during cECG monitoring. Beta-blockers reduce the frequency of ambulatory ischemic events by 60%, reduce their cumulative duration per 48 hours by 70%, and abolish ischemic events in a large minority of patients.[17] This beneficial effect is mediated almost entirely by the heart rate reduction seen with beta-blocker use (Figure 2).[18]
Figure 2.
Graph showing likelihood of developing ischemia after a heart rate increase by the duration and magnitude of the preceding heart rate increase. Likelihood of developing ischemia was directly proportional to both the magnitude and duration of heart rate increases (p<0.001). HR represents heart rate; bpm represents beats per minute. (from Andrews et al. [18])
Calcium channel blockers
In general, diltiazem is effective in the treatment of episodes of ischemia in daily life. However, the heart rate reduction observed during diltiazem treatment is less than that observed during beta-blocker therapy and diltiazem is correspondingly less effective. In the Asymptomatic Cardiac Ischemia Pilot (ACIP) study, the reduction in ischemic episodes was similar using a regimen of either diltiazem or atenolol, as long as the mean heart rate was reduced to a similar degree.[19]
Clinical trials using conventional release nifedipine as a single agent therapy have not generally observed a therapeutic benefit, likely because of vasodilatory effects are counteracted by a reflex-mediated increase in heart rate. The sustained release formulation of nifedipine, which does not cause as significant a reflex tachycardia, reduced the frequency and duration of silent ischemia events by 30%-50% compared to placebo.[20] The addition of amlodipine to a background regimen of a beta-blocker also significantly reduced the frequency and duration of ischemia compared to adding placebo.[21] In the Canadian Amlodipine/Atenolol in Silent Ischemia Study (CASIS), amlodipine alone reduced the frequency of ischemic episodes by 28%, compared to 57% with atenolol alone, and by 72% with a combination regimen.[22] The Circadian Anti-Ischemia Program in Europe (CAPE) II trial compared the efficacy of amlodipine and diltiazem, and the combination of amlodipine/atenolol and diltiazem/isosorbide mononitrate on ischemia during 72-hour cECG monitoring and during exercise testing in patients with stable CAD.[23] Both amlodipine and diltiazem significantly reduced episodes of ambulatory ischemia, with no significant differences between the two treatments. The combination regimen of atenolol/amlodipine produced a highly significant reduction in ambulatory ischemia, while the combination regimen of diltiazem/isosorbide mononitrate showed no significant improvement compared to monotherapy. During exercise testing, the benefits of the combination regimens paralleled the benefits seen during cECG monitoring.
Nitrates
There have been relatively few studies investigating the role of nitrates in the treatment of ischemia during daily life. Von Arnim and Erath compared short and long-acting isosorbide mononitrate and found that each reduced the frequency and duration of episodes of silent ischemia by approximately 70% compared to placebo.[24] Use of a transdermal nitroglycerin patch is associated with a 46% reduction in the daily frequency of episodes of silent ischemia and a 51% reduction in the daily duration of ischemia on the first day of treatment compared to placebo, but the beneficial effect is lost by the second day, even with use of intermittent dosing that included a 12-hour nitrate free period.[25]
Other agents
ACE-inhibitors are known to improve endothelial function, but have only variably shown to reduce ischemia either by cECG or during ETT when compared to placebo.[26, 27] Aspirin and statins have also been shown to reduce episodes of silent ischemia.[28-30] However, Stone et al. also demonstrated that intensive lipid lowering strategies with atorvastatin 80 mg/day in patients with stable coronary disease, a positive exercise treadmill test, a 48-hour cECG with at least one episode of ischemia, and fasting total cholesterol of 180 to 250 mg/dL, did not provide further benefits in ambulatory ischemia, exercise time to onset of ischemia, and angina frequency compared to moderate lipid lowering with diet and low-dose lovastatin.[30]
Studies Targeting cECG Ischemia as a therapeutic goal
The ability of anti-ischemic therapies to improve the adverse prognosis associated with cECG ischemia are inadequately studied. The Atenolol Silent Ischemia Study (ASIST) evaluated 306 patients with ischemia detected by both cECG and exercise tolerance test and found that those patients treated with atenolol had improved event-free survival and increased time to the occurrence of a first adverse event compared with those treated with a placebo.[31] The most powerful multivariable correlate of event-free survival was the absence of cECG ischemia after one month of treatment. The control group in this study, however, received no anti-anginal therapy, and the study consequently does not address the question of the incremental value of treating asymptomatic ischemia detected by cECG monitoring in addition to treating asymptomatic ischemia. In the Total Ischemic Burden Bisoprolol Study (TIBBS),[32] patients whose cECG ischemia was entirely abolished by bisoprolol or nifedipine had a 17.5% event rate at 1 year compared with 32.4% for those who had at least one episode of residual ischemia.
The Asymptomatic Cardiac Ischemia Pilot (ACIP) study[33-35] was designed to determine the feasibility of performing a large-scale clinical trial to assess the prognostic significance of silent ischemia. In the pilot study, 558 patients with coronary anatomy amenable to revascularization, one or more episodes of ischemia on cECG, and ischemia on an exercise test, were randomized to one of three treatment strategies: (1) medication to suppress angina, (2) medication to suppress angina and cECG-measured ischemia, or (3) revascularization. Patients were evaluated with serial cECGs. Medical therapy consisted of atenolol (with long-acting nifedipine, in necessary) or long-acting diltiazem (with isosorbide dinitrate, if necessary). Revascularization was performed with either balloon angioplasty alone (as the study was performed before the modern stent era) or coronary artery bypass surgery. At the 12-week, 6-month, and 1-year follow-up, ischemia was suppressed in each of the three groups, but was more often completely suppressed in patients assigned to revascularization. The escalation of the medical regimens in the ischemia-guided group was, unfortunately, rather minimal, and better ischemia reduction might have been achieved if the dose titration was more aggressive.
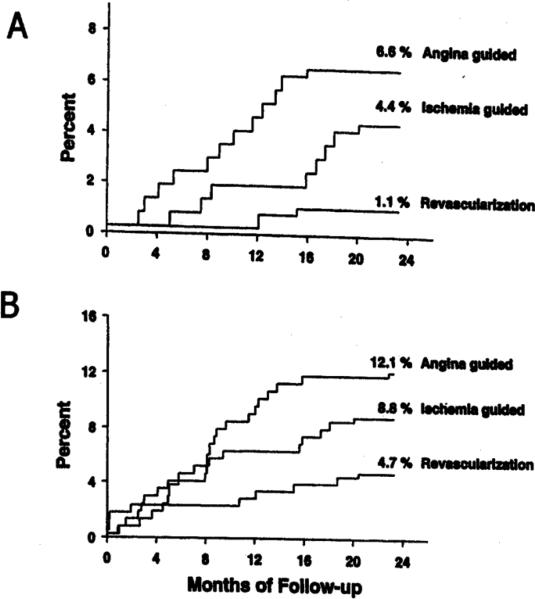
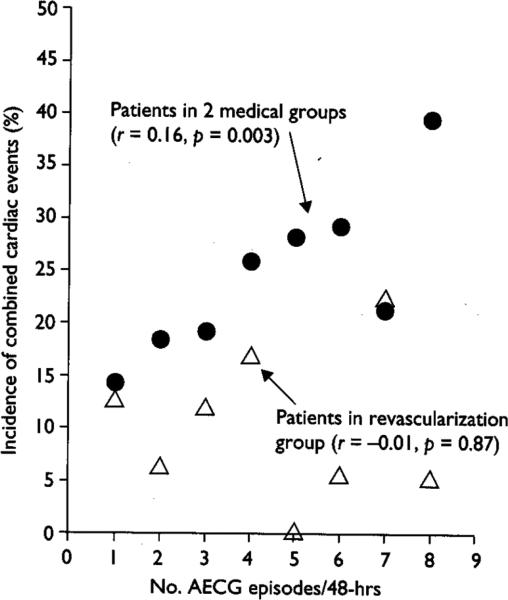
Although not powered to be a prognosis study, the ACIP pilot study provides important insight into the prognostic value of treating cECG-identified ischemia. While the number of fatal events was small, the percentage of patients who died during the 2-year follow up was significantly lower for patients assigned to the revascularization strategy (1.1%) than for those assigned to the angina-guided strategy (6.6%). The ischemia-driven strategy patients had an intermediate mortality rate, though there was no significant difference between the ischemia-guided strategy and the revascularization strategy(Figure 3). Similar results were found combining endpoints for death or MI. In the two medical strategies, there was a trend associating greater reduction in cECG ischemia at 12 weeks with an improved subsequent prognosis (Figure 4). This trend was evident primarily in the ischemia-guided group compared to the angina-guided group. These observations suggest that cECG-guided treatment may enhance prognosis in patients with stable CAD. In contrast to the observations in the two medical groups in ACIP, in the revascularization group there was no association between the number of cECG ischemic episodes at baseline and the incidence of subsequent ischemic events, nor in the change in ischemic episodes from baseline to week 12. These observations suggest that revascularization is effective in lowering the incidence of subsequent events, regardless of the number of ischemic episodes present prior to revascularization, perhaps because the revascularization, especially bypass surgery, bypasses the non-flow limiting plaques (so-called “vulnerable plaques”) as well as the flow-limiting plaques, and thereby improves prognosis from all atherosclerotic plaques.
Figure 3.
Two-year results from the ACIP study. (from Davies et al.,[34])
3A. Two-year cumulative mortality rates for three treatment strategies. Significant differences were seen between revascularization and angina-guided strategies (p<0.005) and between revascularization and ischemia-guided strategies (p<0.05). Angina-guided and ischemia-guided strategies were not significantly different from each other.
3B. Two year cumulative rates of death and MI. Revascularization strategy was significantly different from angina-guided strategy (p<0.01). Differences were not significant between revascularization and ischemia-guided strategies and between angina-guided and ischemia– guided strategies.
Figure 4.
Relationship between cECG ischemic episodes and incidence of combined cardiac events (death, MI, coronary revascularization, or hospitalization for an ischemic event) between week 12 and 1 year. Patients in the two medical groups are indicated by closed circles and patients in the revascularization group are indicated by open triangles. The r values represent the correlation between the number of cECG ischemic episodes and the incidence of combined cardiac events in the respective groups. (from Stone et al. [35]).
ACIP set the stage for the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial.[36] COURAGE randomized 2,287 patients with stable angina to PCI plus optimal medical therapy versus optimal medical therapy alone. COURAGE documented similar composite outcomes between the medical therapy and PCI arms at a median follow-up of 4.6 years. Unfortunately, there was no cECG-ischemia guided arm in COURAGE. Thus, the presence of silent ischemia in the current era still identifies a subset of patients with increased risk of events who should be targeted for more aggressive medical therapy. Further prospective study is still required to address this question, in particular the benefit of revascularization of asymptomatic patients with ischemia detected on cECG. Even in these patients, however, cECG monitoring only identifies the marker of ischemia and not the fundamental adverse prognostic mechanism. Plaque rupture determines adverse outcomes for many patients and cECG does not have the capability to detect these high-risk vulnerable plaques which typically do not obstruct the lumen enough to limit coronary flow and cause ischemic ST-segment depression or angina.
cECG Ischemia in ACS
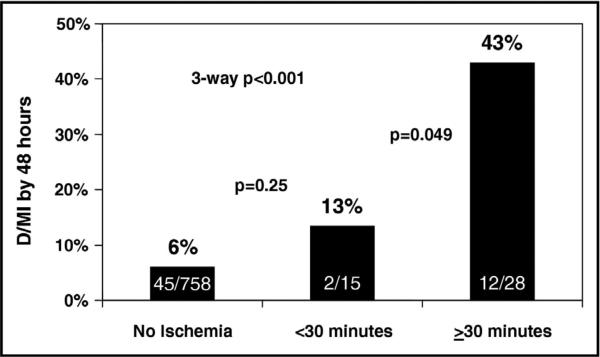
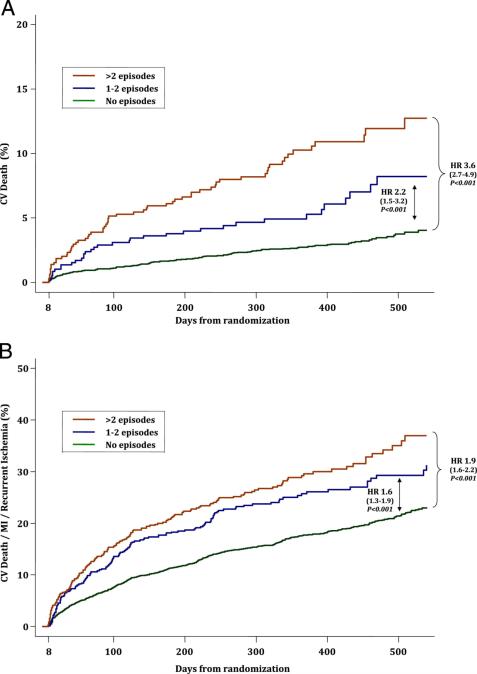
Silent ischemia is known to occur in patients who present with unstable coronary syndromes. [37] The presence of silent ischemia in these patients can provide important prediction of both short-term and long-term risk (Figure 5).[37-41] In patients presenting with unstable angina who were managed medically, evidence of silent ischemia during 48 hours of continuous ECG monitoring was predictive of poor outcomes at both 1-month and 2-years.[42] In a more modern cohort of patients enrolled in the Metabolic Efficiency with Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndrome—Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) trial, most of whom underwent PCI, recurrent ischemia on cECG portended a poor prognosis and was strongly and independently associated with poor cardiovascular outcomes, including cardiovascular death (Figure 6).[40] Similar to patients with stable CAD, patients with unstable angina who had silent ischemia noted by continuous ECG monitoring have been shown to have high-risk coronary anatomy and a corresponding worse prognosis.[43]
Figure 5.
The prognostic importance of ischemia as measured by cECG after percutaneous coronary intervention. D/MI represents the incidence of death or myocardial infarction (from Gibson et al. [41]).
Figure 6.
Ischemia Detected on continuous ECG and outcomes in the MERLIN-TIMI 36 Trial. Cumulative incidence of cardiovascular death (A) and the a composite of cardiovascular death, myocardial infarction (MI), or recurrent ischemia (B) according to the number of ischemic episodes detected on continuous ECG. The red lines represent >2 episodes; the blue lines represent 1 to 2 episodes; the green lines represent no episodes. HR represents hazard ratio; CV represents cardiovascular; MI represents myocardial infarction. (from Scirica et al. [40]).
More recently, a new metric on cECG, termed morphologic variability, has been used to identify those at high risk after NSTEMI. Morphologic variability measures beat-to-beat variability in the shape of the entire heart beat signal. This metric is analogous to heart rate variability, which focuses on beat-to-beat changes in the heart rate. In the Dose confirmation Study assessing anti-Platelet Effects of AZD6140 vs. clopidogrel in non–ST-segment Elevation myocardial infarction-2 (DISPERSE-2) trial, patients with high morphologic variability showed an increased risk of death during follow-up (hazard ratio 8.46; p <0.001).[44] The relationship between high morphologic variability and death could be observed even after adjusting for baseline clinical characteristics and heart rate variability measures (adjusted hazard ratio 6.91; p = 0.001).
In acute myocardial infarction, risk stratification with continuous ECG can also be useful. In 406 patients with acute myocardial infarction who were assessed with cECG 5-7 days after the infarct, recurrent ischemia detected by ECG was one of the most powerful predictors of adverse events. [45]
Episodes of transient ST-segment deviation have also been used to assess therapeutic efficacy in a variety of interventions across the spectrum of ACS. In the c7E3 Fab Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) study in patients with unstable angina, asymptomatic recurrent ischemia was detected in 18% of abciximab-treated patients and in 23% of placebo-treated patients. Abciximab also reduced the total ischemic burden.[46] In a small study of 100 patients with ACS randomized to either atorvastatin 80 mg/day or placebo, there was a trend towards lower risk of developing recurrent of prolonged episodes of ST-segment deviation during 48-hour continuous ECG monitoring in patients treated with atorvastatin.[47]
In STEMI, the speed with which ST-segment elevation returns to baseline, a metric readily assessed by cECG, has long been a hallmark of successful reperfusion with either thrombolytic therapy or PCI and has been associated with improved myocardial tissue perfusion and improved prognosis.[48]
Future Directions
cECG monitoring may be useful in other patient populations. In patients who have undergone heart transplantation, coronary allograft vasculopathy is a common clinical problem.[49] As these patients have denervated hearts, they rarely present with typical angina symptoms, instead often presenting with heart failure of decrements in left ventricular ejection fraction after multiple small infarcts. Targeted use of cECG monitoring in these patients for silent ischemia may fruitful and further research is warranted.
Recently, an implantable ECG monitor has been described. The AngelMed Guardian ischemia detection system (Angel Medical Systems, Shrewsbury, NJ) places a pacemaker lead at the right ventricular apex and continuously monitors intracardiac electrograms.[50] This system alerts the patient to abnormal ST-segment elevation, and initial clinical data suggest an improvement in the time-to-presentation after the development of a clinical ischemic event. Future development of this system, or other technologies, may allow improvements in the delivery of clinical care by providing physiologic data to patients and physicians can rapidly make adjustments to patient medical regimens or provide for more timely urgent revascularization.
Summary and Conclusions
Asymptomatic ischemia is common in patients with stable CAD during routine daily activities. Since asymptomatic ischemia is, by definition, not associated with any symptoms or discomfort, the detection of ischemia by cECG would only be of clinical significance if its presence was independently associated with adverse prognosis. Observational studies and small-scale RCTs suggest that more aggressive and thorough treatment of ischemia would lead to an improved outcome, but more definitive large-scale study is necessary to confirm this relationship. Identification of ischemia by cECG monitoring across the spectrum of ACS indicates high risk patient subsets, similar to other modalities. In patients with acute MI, continuous ECG monitoring can be used to gauge the efficacy of treatment by determining the resolution of ST-segment elevation.
Future research in this area is warranted to study the effect of newer medical therapies or procedures and to evaluate whether therapy guided toward silent ischemia will improve patient outcomes.
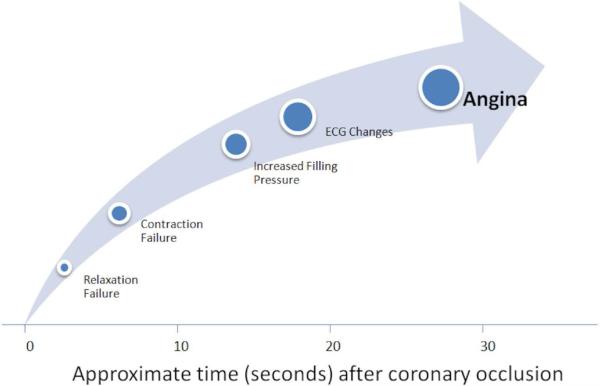
Figure 1.
Following coronary artery occlusion diastolic, systolic, and ECG changes precede the development of angina (from Conti et al. [8])
Acknowledgments
Dr. Wimmer was supported in part by NIH T32-HL007604.
Dr. Scirica is employed by the TIMI Study Group/Brigham and Women's Hospital, which has received research grants from Abbott, AstraZeneca, Amgen, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Daichii Sankyo, Eisai, Eli Lilly, Gilead, GlaxoSmithKline, Merck (SPRI), Novartis, Pfizer, Roche Diagnostics, Sanofi-Aventis, and Johnson & Johnson, as well as grant support through Brigham and Women's Hospital from Brahams, Critical Diagnostics, Genzyme, Nanosphere, and Takeda. Dr. Scirica has also served as a consultant for Lexicon, Arena, Gilead, Eisai, and St. Jude's Medical.
Abbreviations
- cECG
continuous electrocardiogram
- AECG
Ambulatory electrocardiogram
- CAD
coronary artery disease
- RCT
randomized clinical trial
- PCI
percutaneous coronary intervention
- NSTEMI
non-ST-segment elevation myocardial infarction
- STEMI
ST-segment elevation myocardial infarction
Footnotes
Conflicts/Disclosures: Dr. Wimmer and Dr. Stone have no conflicts of interest to report.
References
- 1.Stern S, Tzivoni D. Early detection of silent ischaemic heart disease by 24-hour electrocardiographic monitoring of active subjects. Br Heart J. 1974;36(5):481–6. doi: 10.1136/hrt.36.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schang SJ, Jr., Pepine CJ. Transient asymptomatic S-T segment depression during daily activity. Am J Cardiol. 1977;39(3):396–402. doi: 10.1016/s0002-9149(77)80095-7. [DOI] [PubMed] [Google Scholar]
- 3.Deedwania PC, Carbajal EV. Silent ischemia during daily life is an independent predictor of mortality in stable angina. Circulation. 1990;81(3):748–56. doi: 10.1161/01.cir.81.3.748. [DOI] [PubMed] [Google Scholar]
- 4.Chipkin SR, Frid D, Alpert JS, et al. Frequency of painless myocardial ischemia during exercise tolerance testing in patients with and without diabetes mellitus. Am J Cardiol. 1987;59(1):61–5. doi: 10.1016/s0002-9149(87)80070-x. [DOI] [PubMed] [Google Scholar]
- 5.Marchant B, Umachandran V, Stevenson R, et al. Silent myocardial ischemia: role of subclinical neuropathy in patients with and without diabetes. J Am Coll Cardiol. 1993;22(5):1433–7. doi: 10.1016/0735-1097(93)90554-e. [DOI] [PubMed] [Google Scholar]
- 6.Rosen SD, Paulesu E, Nihoyannopoulos P, et al. Silent ischemia as a central problem: regional brain activation compared in silent and painful myocardial ischemia. Ann Intern Med. 1996;124(11):939–49. doi: 10.7326/0003-4819-124-11-199606010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Stone PH, Chaitman BR, McMahon RP, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) Study. Relationship between exercise-induced and ambulatory ischemia in patients with stable coronary disease. Circulation. 1996;94(7):1537–44. doi: 10.1161/01.cir.94.7.1537. [DOI] [PubMed] [Google Scholar]
- 8.Conti CR, Bavry AA, Petersen JW. Silent ischemia: clinical relevance. J Am Coll Cardiol. 2012;59(5):435–41. doi: 10.1016/j.jacc.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Smith SC, Jr., Amsterdam E, Balady GJ, et al. Prevention Conference V: Beyond secondary prevention: identifying the high-risk patient for primary prevention: tests for silent and inducible ischemia: Writing Group II. Circulation. 2000;101(1):E12–6. doi: 10.1161/01.cir.101.1.e12. [DOI] [PubMed] [Google Scholar]
- 10.Cohn PF, Fox KM, Daly C. Silent myocardial ischemia. Circulation. 2003;108(10):1263–77. doi: 10.1161/01.CIR.0000088001.59265.EE. [DOI] [PubMed] [Google Scholar]
- 11.Deedwania PC, Nelson JR. Pathophysiology of silent myocardial ischemia during daily life. Hemodynamic evaluation by simultaneous electrocardiographic and blood pressure monitoring. Circulation. 1990;82(4):1296–304. doi: 10.1161/01.cir.82.4.1296. [DOI] [PubMed] [Google Scholar]
- 12.Pepine CJ, Geller NL, Knatterud GL, et al. The Asymptomatic Cardiac Ischemia Pilot (ACIP) study: design of a randomized clinical trial, baseline data and implications for a long-term outcome trial. J Am Coll Cardiol. 1994;24(1):1–10. doi: 10.1016/0735-1097(94)90534-7. [DOI] [PubMed] [Google Scholar]
- 13.Stramba-Badiale M, Bonazzi O, Casadei G, et al. Prevalence of episodes of ST-segment depression among mild-to-moderate hypertensive patients in northern Italy: the Cardioscreening Study. J Hypertens. 1998;16(5):681–8. doi: 10.1097/00004872-199816050-00016. [DOI] [PubMed] [Google Scholar]
- 14.Milan Study on Atherosclerosis and Diabetes (MiSAD) Group Prevalence of unrecognized silent myocardial ischemia and its association with atherosclerotic risk factors in noninsulin-dependent diabetes mellitus. Am J Cardiol. 1997;79(2):134–9. doi: 10.1016/s0002-9149(96)00699-6. [DOI] [PubMed] [Google Scholar]
- 15.Cosson E, Guimfack M, Paries J, et al. Are silent coronary stenoses predictable in diabetic patients and predictive of cardiovascular events? Diabetes Metab. 2003;29(5):470–6. doi: 10.1016/s1262-3636(07)70060-5. [DOI] [PubMed] [Google Scholar]
- 16.Rocco MB, Nabel EG, Campbell S, et al. Prognostic importance of myocardial ischemia detected by ambulatory monitoring in patients with stable coronary artery disease. Circulation. 1988;78(4):877–84. doi: 10.1161/01.cir.78.4.877. [DOI] [PubMed] [Google Scholar]
- 17.Deedwania PC, Carbajal EV. Prevalence and patterns of silent myocardial ischemia during daily life in stable angina patients receiving conventional antianginal drug therapy. Am J Cardiol. 1990;65(16):1090–6. doi: 10.1016/0002-9149(90)90319-v. [DOI] [PubMed] [Google Scholar]
- 18.Andrews TC, Fenton T, Toyosaki N, et al. Subsets of ambulatory myocardial ischemia based on heart rate activity. Circadian distribution and response to anti-ischemic medication. The Angina and Silent Ischemia Study Group (ASIS). Circulation. 1993;88(1):92–100. doi: 10.1161/01.cir.88.1.92. [DOI] [PubMed] [Google Scholar]
- 19.Pratt CM, McMahon RP, Goldstein S, et al. Comparison of subgroups assigned to medical regimens used to suppress cardiac ischemia (the Asymptomatic Cardiac Ischemia Pilot [ACIP] Study). Am J Cardiol. 1996;77(15):1302–9. doi: 10.1016/s0002-9149(96)00196-8. [DOI] [PubMed] [Google Scholar]
- 20.Parmley WW, Nesto RW, Singh BN, et al. Attenuation of the circadian patterns of myocardial ischemia with nifedipine GITS in patients with chronic stable angina. N-CAP Study Group. J Am Coll Cardiol. 1992;19(7):1380–9. doi: 10.1016/0735-1097(92)90591-a. [DOI] [PubMed] [Google Scholar]
- 21.Deanfield JE, Detry JM, Lichtlen PR, et al. Amlodipine reduces transient myocardial ischemia in patients with coronary artery disease: double-blind Circadian Anti-Ischemia Program in Europe (CAPE Trial). J Am Coll Cardiol. 1994;24(6):1460–7. doi: 10.1016/0735-1097(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 22.Davies RF, Habibi H, Klinke WP, et al. Effect of amlodipine, atenolol and their combination on myocardial ischemia during treadmill exercise and ambulatory monitoring. Canadian Amlodipine/Atenolol in Silent Ischemia Study (CASIS) Investigators. J Am Coll Cardiol. 1995;25(3):619–25. doi: 10.1016/0735-1097(94)00436-t. [DOI] [PubMed] [Google Scholar]
- 23.Deanfield JE, Detry JM, Sellier P, et al. Medical treatment of myocardial ischemia in coronary artery disease: effect of drug regime and irregular dosing in the CAPE II trial. J Am Coll Cardiol. 2002;40(5):917–25. doi: 10.1016/s0735-1097(02)02050-8. [DOI] [PubMed] [Google Scholar]
- 24.von Arnim T, Erath A. Nitrates and calcium antagonists for silent myocardial ischemia. Am J Cardiol. 1988;61(9):15E–8E. doi: 10.1016/0002-9149(88)90083-5. [DOI] [PubMed] [Google Scholar]
- 25.Nabel EG, Barry J, Rocco MB, et al. Effects of dosing intervals on the development of tolerance to high dose transdermal nitroglycerin. Am J Cardiol. 1989;63(11):663–9. doi: 10.1016/0002-9149(89)90248-8. [DOI] [PubMed] [Google Scholar]
- 26.Pepine CJ, Rouleau JL, Annis K, et al. Effects of angiotensin-converting enzyme inhibition on transient ischemia: the Quinapril Anti-Ischemia and Symptoms of Angina Reduction (QUASAR) trial. J Am Coll Cardiol. 2003;42(12):2049–59. doi: 10.1016/j.jacc.2003.07.027. [DOI] [PubMed] [Google Scholar]
- 27.Tzivoni D, Gottlieb S, Khurmi NS, et al. Effect of benazepril on myocardial ischaemia in patients with chronic stable angina pectoris. Eur Heart J. 1992;13(8):1129–34. doi: 10.1093/oxfordjournals.eurheartj.a060325. [DOI] [PubMed] [Google Scholar]
- 28.Mahony C. Effect of aspirin on myocardial ischemia. Am J Cardiol. 1989;64(5):387–9. doi: 10.1016/0002-9149(89)90541-9. [DOI] [PubMed] [Google Scholar]
- 29.van Boven AJ, Jukema JW, Zwinderman AH, et al. Reduction of transient myocardial ischemia with pravastatin in addition to the conventional treatment in patients with angina pectoris. REGRESS Study Group. Circulation. 1996;94(7):1503–5. doi: 10.1161/01.cir.94.7.1503. [DOI] [PubMed] [Google Scholar]
- 30.Stone PH, Lloyd-Jones DM, Kinlay S, et al. Effect of intensive lipid lowering, with or without antioxidant vitamins, compared with moderate lipid lowering on myocardial ischemia in patients with stable coronary artery disease: the Vascular Basis for the Treatment of Myocardial Ischemia Study. Circulation. 2005;111(14):1747–55. doi: 10.1161/01.CIR.0000160866.90148.76. [DOI] [PubMed] [Google Scholar]
- 31.Pepine CJ, Cohn PF, Deedwania PC, et al. Effects of treatment on outcome in mildly symptomatic patients with ischemia during daily life. The Atenolol Silent Ischemia Study (ASIST). Circulation. 1994;90(2):762–8. doi: 10.1161/01.cir.90.2.762. [DOI] [PubMed] [Google Scholar]
- 32.Von Arnim T. Prognostic significance of transient ischemic episodes: response to treatment shows improved prognosis. Results of the Total Ischemic Burden Bisoprolol Study (TIBBs) follow-up. J Am Coll Cardiol. 1996;28(1):20–4. doi: 10.1016/0735-1097(96)00122-2. [DOI] [PubMed] [Google Scholar]
- 33.Rogers WJ, Bourassa MG, Andrews TC, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study: outcome at 1 year for patients with asymptomatic cardiac ischemia randomized to medical therapy or revascularization. The ACIP Investigators. J Am Coll Cardiol. 1995;26(3):594–605. doi: 10.1016/0735-1097(95)00228-v. [DOI] [PubMed] [Google Scholar]
- 34.Davies RF, Goldberg AD, Forman S, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study two-year follow-up: outcomes of patients randomized to initial strategies of medical therapy versus revascularization. Circulation. 1997;95(8):2037–43. doi: 10.1161/01.cir.95.8.2037. [DOI] [PubMed] [Google Scholar]
- 35.Stone PH, Chaitman BR, Forman S, et al. Prognostic significance of myocardial ischemia detected by ambulatory electrocardiography, exercise treadmill testing, and electrocardiogram at rest to predict cardiac events by one year (the Asymptomatic Cardiac Ischemia Pilot [ACIP] study). Am J Cardiol. 1997;80(11):1395–401. doi: 10.1016/s0002-9149(97)00706-6. [DOI] [PubMed] [Google Scholar]
- 36.Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 37.Gottlieb SO, Weisfeldt ML, Ouyang P, et al. Silent ischemia as a marker for early unfavorable outcomes in patients with unstable angina. N Engl J Med. 1986;314(19):1214–9. doi: 10.1056/NEJM198605083141903. [DOI] [PubMed] [Google Scholar]
- 38.Langer A, Singh N, Freeman MR, et al. Detection of silent ischemia adds to the prognostic value of coronary anatomy and left ventricular function in predicting outcome in unstable angina patients. Can J Cardiol. 1995;11(2):117–22. [PubMed] [Google Scholar]
- 39.Patel DJ, Knight CJ, Holdright DR, et al. Pathophysiology of transient myocardial ischemia in acute coronary syndromes. Characterization by continuous ST-segment monitoring. Circulation. 1997;95(5):1185–92. doi: 10.1161/01.cir.95.5.1185. [DOI] [PubMed] [Google Scholar]
- 40.Scirica BM, Morrow DA, Budaj A, et al. Ischemia detected on continuous electrocardiography after acute coronary syndrome: observations from the MERLIN-TIMI 36 (Metabolic Efficiency With Ranolazine for Less Ischemia in Non-ST-Elevation Acute Coronary Syndrome-Thrombolysis In Myocardial Infarction 36) trial. J Am Coll Cardiol. 2009;53(16):1411–21. doi: 10.1016/j.jacc.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 41.Gibson CM, Pride YB, Buros JL, et al. Timing and duration of myocardial ischemia on Holter monitoring following percutaneous coronary intervention and their association with clinical outcomes (a PROTECT-TIMI 30 Substudy Analysis). Am J Cardiol. 2009;104(1):36–40. doi: 10.1016/j.amjcard.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb SO, Weisfeldt ML, Ouyang P, et al. Silent ischemia predicts infarction and death during 2 year follow-up of unstable angina. J Am Coll Cardiol. 1987;10(4):756–60. doi: 10.1016/s0735-1097(87)80267-x. [DOI] [PubMed] [Google Scholar]
- 43.Patel DJ, Gomma AH, Knight CJ, et al. Why is recurrent myocardial ischaemia a predictor of adverse outcome in unstable angina? An observational study of myocardial ischaemia and its relation to coronary anatomy. Eur Heart J. 2001;22(21):1991–6. doi: 10.1053/euhj.2001.2680. [DOI] [PubMed] [Google Scholar]
- 44.Syed Z, Scirica BM, Mohanavelu S, et al. Relation of death within 90 days of non-ST-elevation acute coronary syndromes to variability in electrocardiographic morphology. Am J Cardiol. 2009;103(3):307–11. doi: 10.1016/j.amjcard.2008.09.099. [DOI] [PubMed] [Google Scholar]
- 45.Gill JB, Cairns JA, Roberts RS, et al. Prognostic importance of myocardial ischemia detected by ambulatory monitoring early after acute myocardial infarction. N Engl J Med. 1996;334(2):65–70. doi: 10.1056/NEJM199601113340201. [DOI] [PubMed] [Google Scholar]
- 46.Klootwijk P, Meij S, Melkert R, et al. Reduction of recurrent ischemia with abciximab during continuous ECG-ischemia monitoring in patients with unstable angina refractory to standard treatment (CAPTURE). Circulation. 1998;98(14):1358–64. doi: 10.1161/01.cir.98.14.1358. [DOI] [PubMed] [Google Scholar]
- 47.Correia LC, Magalhaes LP, Santana O, et al. Effect of atorvastatin (80 mg) on recurrent ischemia in unstable angina pectoris or non-ST-elevation acute myocardial infarction. Am J Cardiol. 2003;91(11):1355–7. doi: 10.1016/s0002-9149(03)00330-8. [DOI] [PubMed] [Google Scholar]
- 48.de Lemos JA, Braunwald E. ST segment resolution as a tool for assessing the efficacy of reperfusion therapy. J Am Coll Cardiol. 2001;38(5):1283–94. doi: 10.1016/s0735-1097(01)01550-9. [DOI] [PubMed] [Google Scholar]
- 49.Lee MS, Finch W, Weisz G, et al. Cardiac allograft vasculopathy. Rev Cardiovasc Med. 2011;12(3):143–52. [PubMed] [Google Scholar]
- 50.Fischell TA, Fischell DR, Avezum A, et al. Initial clinical results using intracardiac electrogram monitoring to detect and alert patients during coronary plaque rupture and ischemia. J Am Coll Cardiol. 2010;56(14):1089–98. doi: 10.1016/j.jacc.2010.04.053. [DOI] [PubMed] [Google Scholar]