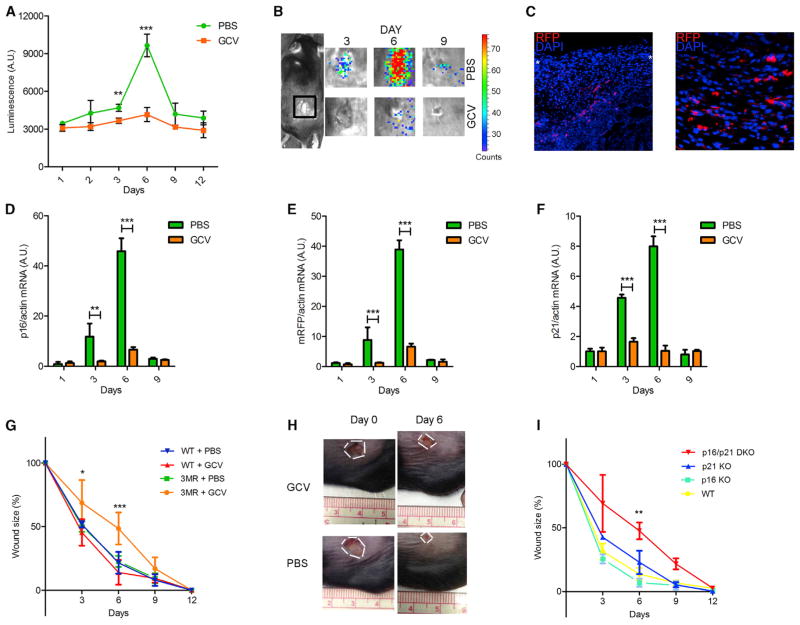
Figure 3. Senescent Cells Are Induced and Necessary for Optimal Cutaneous Wound Healing.
In all cases, mice were wounded using a 6 mm punch to dorsal skin and treated with PBS (vehicle control) or GCV (five daily i.p. injections) from 1 to 6 days after injury (n = at least 4 mice per group).
(A and B) p16-3MR mice were wounded, injected i.p. with coelentarazine, and imaged with a Xenogen imaging system at the indicated times after injury. (A) Quantification of the luminescence. (B) Typical images, both at the indicated time (days) after injury.
(C) Skin biopsies of p16-3MR mice were collected 6 days after injury, fixed, and stained for nuclei (DAPI; blue) or mRFP (immunostaining; red). White asterisks define the wound edges.
(D–F) p16INK4a (D), mRFP (E), and p21 (F) mRNA levels were quantified by qRT-PCR from skin biopsies excised from PBS or GCV-treated wounds to p16-3MR mice at the indicated intervals after injury. Actin was used to control for cDNA quantity.
(G) Wound sizes of WT or p16-3MR mice were measured at the indicated days after wounding.
(H) Representative image of wounds at the indicated days after injury of p16-3MR mice.
(I) Wound sizes of WT, p16 KO, p21 KO, and p16/p21 DKO mice were measured at the indicated days after wounding.
Data shown are the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.0001.