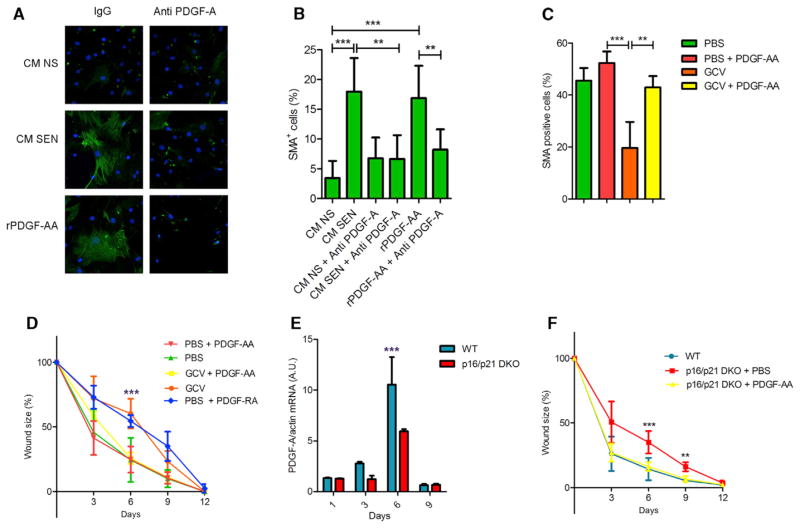
Figure 6. Senescence-Associated PDGF-A Drives Myofibroblast Differentiation.
(A and B) Skin fibroblasts were incubated with conditioned media from IR-induced senescent (CM SEN) or nonsenescent (CM NS) cells containing nonspecific rabbit IgG or a PDGF-A blocking antibody; after 48 hr, the cells were immunostained for SMA (green), and nuclei were stained with DAPI (blue). Ten ng/ml recombinant PDGF-AA was used as a positive control. (A) Representative image. (B) Percentage of SMA-positive cells relative to the total (DAPI positive) number of cells (n = 6).
(C and D) Wound healing was performed as described in Figure 3G, except PDGF-AA (20 ng) or vehicle (PBS) were topically applied daily from 1 to 6 d after wounding (n = 5). (C) Biopsies from wounds 6 days after injury were embedded in paraffin and stained for SMA. The graph shows the percentage of positive cells present in the wound gap. (D) Wound sizes were measured at the indicated times after injury. In addition to the groups described, a cohort of control p16-3MR mice was treated with PDGF-RA (daily topical application of 20 ng from 1 to 6 days after injury).
(E) RNA was isolated from wounded areas 6 days after injury of WT and p16/p21 DKO mice. PDGFA mRNA levels were quantified by qRT-PCR. Actin was used to control for RNA quantity (n = 3).
(F) Wound healing was performed and measured as described in (B). PDGF-AA (20 ng) or vehicle (PBS) were topically applied daily from 1 to 6 days after wounding (n = 3). Data shown are the mean ± SD. **p < 0.01, ***p < 0.0001.