Abstract
Background
Previous reports have demonstrated similar survival for men and women on hemodialysis, despite women’s increased survival in the general population.
Objectives
To examine the effect of age on mortality in women undergoing chronic hemodialysis.
Design
A retrospective cohort study using an administrative data registry, the Canadian Organ Replacement Registry (CORR) from Jan. 2001 and Dec. 2009.
Setting
Canada.
Patients
28,971 (Women 11,792 (40.7%), Men 17,179 (59.3%)) incident chronic hemodialysis patients who survived greater than 90 days on dialysis.
Measurements
All-cause mortality.
Methods
Cox proportional hazards and competing risks models were employed to determine the independent association between sex, age and likelihood of all-cause mortality with renal transplantation as the competing outcome.
Results
During the study period, 6060 (51.4%) of women and 8650 (50.4%) of men initiating dialysis died. Younger women experienced higher mortality (Age < 45: Women 22.5%, Men 18.2%, hazard ratio (HR) 1.31 (1.12-1.52)) whereas elderly women experience lower mortality (Age 75–85: Women 65%, Men 67.3%, HR 0.94 95% CI 0.88-0.99, Age > 85: Women 66%, Men 70.2%, HR 0.83 95% CI 0.71-0.97) compared to men. This relationship persisted after accounting for the competing risk of transplantation.
Limitations
The cause of death was unknown.
Conclusions
Women’s survival on chronic hemodialysis varies by age compared to men with a significantly higher mortality in women younger than 45 years old and lower mortality in woman older than 75 years of age.
Abrégé
Contexte
Des rapports précédents ont démontré une survie similaire pour les hommes et les femmes en hémodialyse, malgré une meilleure survie pour les femmes dans la population générale.
Objectifs
Examiner l’incidence de l’âge sur la mortalité chez les femmes en hémodialyse chronique.
Type d’étude
Une étude de cohorte rétrospective basée sur un registre de données administratives, le Registre canadien des insuffisances et des transplantations d’organes (RCITO), de janvier 2001 à décembre 2009.
Échantillon
Canada
Participants
28 971 (femmes 11 792 (40,7%), hommes 17 179 (59,3%)) nouveaux patients souffrant d’insuffisance rénale et traités en hémodialyse, qui ont survécu plus de 90 jours.
Mesures
Mortalité toutes causes confondues.
Méthodes
On a eu recours au modèle des risques proportionnels de Cox et au modèle de probabilités concurrentes pour déterminer l’association indépendante entre le genre, l’âge et les probabilités de mortalité toutes causes confondues, et la transplantation rénale est considérée comme un résultat concurrent.
Résultats
Au cours de la période d’étude, 6 060 (51,4%) des femmes et 8 650 (50,4%) des hommes qui ont entamé la dialyse sont décédés. La mortalité des jeunes femmes a été supérieure (âge < 45: femmes 22,5%, hommes 18,2%, rapport des risques (hazard ratio, HR) 1,31 (1,12-1,52)), alors que la mortalité des femmes âgées a été inférieure (âge 75-85: femmes 65%, hommes 67,3%, HR 0,9495% IC 0,88-0,99; âge >85: femmes 66%, hommes 70,2%, HR 0,83 95% IC 0,71-0,97) que celle des hommes. Ce rapport demeurait, après avoir pris en considération le risque concurrent de la transplantation.
Limites de l’étude
La cause du décès est inconnue.
Conclusions
La survie des femmes en hémodialyse chronique, comparativement à celle des hommes, varie selon l'âge. En effet, la mortalité des femmes de moins de 45 ans est significativement supérieure à celle des hommes du même âge alors que celle des femmes âgées de plus de 75 ans est inférieure à celle des hommes de plus de 75 ans.
What was known before
Women in the general population experience increased longevity relative to men. Furthermore previous reports have suggested higher mortality in younger woman on hemodialysis. Whether this is true in a Canadian population, after accounting for multiple confounders and the competing risk of kidney transplantation, remains unknown.
What this adds
We found that women’s survival on chronic hemodialysis varies by age with higher mortality in women younger than 45 years old and lower mortality in woman older than 75 years of age compared to men and this was persistent after accounting for the competing risk of transplantation.
Background
Women comprise a significant and increasing proportion of the worldwide dialysis population. Several studies have identified significant differences in prevalence and treatment of kidney disease between the sexes. Women appear less likely than men to start dialysis for treatment of kidney failure and less likely to receive a kidney transplant [1–4]. Upon initiation of dialysis, women are more likely to receive a lower dialysis dose, inferior treatment of anemia and mineral metabolism complications, and have a catheter as a vascular access [5–7]. Although, attempts to mitigate sex-based differences in renal care have been attempted; significant disparities still exist [5].
Despite clearly documented differences in the delivery of dialysis care, the questions of whether these differences adversely impact the outcome of women on dialysis remains unclear. A small number of previous studies suggest younger women on hemodialysis may have a higher mortality relative to men [2, 8, 9]. Furthermore, elderly women have been reported to have comparable survival to elderly men on hemodialysis [2, 8, 9]. Whether these findings are applicable to the Canadian dialysis population remains unknown. In populations with disparate rates of censoring and competing events, such as differences in transplantation in end stage renal disease (ESRD), appropriate analyses must be employed to account for these differences [10, 11]. The use of competing outcomes analyses has allowed considerable insight into sex based disparities in transplantation rates on hemodialysis demonstrating elderly women are less likely to receive a renal transplant and young African Americans on hemodialysis experience a higher mortality [3, 12]. Relatively healthy elderly women may remain on hemodialysis, as opposed to healthy elderly men who may receive a transplant, creating a survival disparity. Accounting for the competing event of transplantation may alter the survival estimates of elderly women on hemodialysis such that it mimics observations from the general population, where women experience a survival benefit and improved longevity compared to men.
In this regard we set out to examine sex-based differences on mortality among a chronic hemodialysis cohort utilizing a competing risk analysis and whether age was an effect modifier. We hypothesized that women would experience higher survival after accounting for the competing risk of transplantation and this would differ by age.
Results
Sex and age-based patient characteristics
Women were the minority on hemodialysis therapies across all age categories (Table 1). They comprised a larger proportion of the hemodialysis population among Aboriginal and East Asian’s. Older women (>45 years of age) had a higher BMI and shorter pre-dialysis care than men. Older men (>45 years of age) had more cardiac and vascular disease, were more likely to be current cigarette smokers and have a higher number of co-morbid illnesses. Women were more likely to have interstitial disease as the etiology of ESRD, and had a lower serum albumin and hemoglobin. Younger women (<45 years or age) were more likely to have pulmonary edema and malignancy at dialysis initiation and less likely to be on an anti-hypertensive medication. Lastly women were significantly less likely to initiate hemodialysis with an arteriovenous fistula (AVF) or arteriovenous graft (AVG) across all age groups with absolute difference of 4.0 to 5.7%.
Table 1.
Characteristics of the study cohort stratified by sex and age categories
| Characteristic | Age < 45 | Age 45-75 | Age > 75 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | P value | Women | Men | P value | Women | Men | P value | |
| N % | 39.5(1406) | 60.5(2152) | 40.0(7044) | 60.0(10585) | 42.9(3342) | 57.1(4442) | |||
| Age (±SD) | 35.5 ± 7.4 | 35.9 ± 7.2 | 0.1 | 63.4 ± 8.2 | 62.9 ± 8.3 | <0.0001 | 81.0 ± 4.0 | 80.9 ± 4.0 | 0.5 |
| Race | <0.0001 | <0.0001 | 0.02 | ||||||
| Caucasian | 57.6(810) | 65.1(1400) | 70(4856) | 74(7490) | 77.8(2599) | 80.6(3579) | |||
| Aboriginal | 15.9(223) | 10.3(222) | 7.3(644) | 4.8(635) | 1.6(53) | 1.1(51) | |||
| East Asian | 6.8(95) | 5.3(115) | 5.1(359) | 6.6(541) | 7.8(260) | 6.2(275) | |||
| Black | 6.1(86) | 5.4(116) | 3.4(724) | 2.8(1234) | 9.2(306) | 8.9(397) | |||
| South Asian | 9.2(129) | 9.3(201) | 11.1(255) | 9.1(345) | 1.7(57) | 1.3(58) | |||
| Other | 4.5(63) | 4.6(98) | 3.1(206) | 2.7(340) | 2.0(67) | 1.8(82) | |||
| BMI (±SD) | 27.2 ± 8.6 | 26.9 ± 6.5 | 0.2 | 28.9 ± 8.1 | 27.9 ± 6.4 | <0.0001 | 25.8 ± 6.1 | 25.4 ± 5.3 | <0.0001 |
| Distance to nearest dialysis facility in km (IQR) | 11.4(5.0-62.2) | 11.7(4.8-62.4) | 0.7 | 12.0(4.9-59.5) | 12.0(5.0-55.5) | 0.9 | 8.6(4.0-28.8) | 9.4(4.4-34.8) | 0.06 |
| Any pre-dialysis care % (N) | 53.2(748) | 50.9(1096) | 0.2 | 58.5(4122) | 58.7(6212) | 0.8 | 57.2(1911) | 59.2(2630) | 0.07 |
| Median number of days with pre-dialysis care (IQR) | 562(220–1532) | 574(211–1339) | 0.9 | 133 (0–747) | 140(0–812) | 0.03 | 587 (217–1218) | 666(246–1362) | 0.003 |
| Geographic region % (N) | 0.08 | 0.06 | 0.2 | ||||||
| Atlantic | 9.2(129) | 9.4(202) | 11.6(815) | 10.8(1137) | 10.3(342) | 10.4(461) | |||
| Central | 19.3(685) | 31.6(1122) | 51.6(3626) | 53.2(5618) | 59.2(1969) | 57.7(2558) | |||
| Prairie | 12(425) | 15.9(566) | 24.3(1705) | 23.2(2447) | 17.6(586) | 19.5(863) | |||
| Pacific | 11.8(166) | 12.0(258) | 12.5(882) | 12.9(1367) | 13(431) | 12.5(555) | |||
| Co-morbidities: % (N) | |||||||||
| CAD | 5.1(72) | 5.6(120) | 0.6 | 22.3(1572) | 23.7(2508) | 0.04 | 26.4(881) | 29.1(1292) | 0.008 |
| AMI | 3.4(48) | 3.8(82) | 0.6 | 19.4(1370) | 25.2(2666) | <0.0001 | 22.9(765) | 30.3(1344) | <0.0001 |
| Pulmonary edema | 14.4(202) | 11.6(249) | 0.02 | 29.5(2080) | 26.8(2837) | <0.0001 | 32(1070) | 30.5(1357) | 0.2 |
| DM | 35.2(495) | 36(774) | 0.7 | 56.6(3984) | 55.1(5932) | 0.06 | 38.3(1279) | 36.1(1602) | 0.05 |
| Stroke | 3.8(54) | 3.3(70) | 0.4 | 14.0(988) | 14.6(1541) | 0.3 | 16.7(557) | 19.5(865) | 0.002 |
| PVD | 6.7(94) | 6.7(144) | 0.9 | 17.9(1263) | 22.4(2370) | <0.0001 | 17.7(590) | 23.8(1055) | <0.0001 |
| Malignancy | 3.4(48) | 1.8(38) | 0.002 | 10.7(757) | 11.5(1217) | 0.2 | 15(500) | 21.5(957) | <0.0001 |
| Lung disease | 3.4(48) | 2.3(49) | 0.05 | 12.7(894) | 11.5(1215) | 0.02 | 12.1(405) | 15.4(683) | <0.0001 |
| HTN | 71.3(1003) | 77.6(1670) | <0.0001 | 83.3(5870) | 83.2(8803) | 0.8 | 84.4(2819) | 82(3642) | 0.006 |
| Serious illness | 9.7(136) | 8.5(183) | 0.3 | 11.3(794) | 11.3(1198) | 0.9 | 9.4(314) | 9.8(437) | 0.5 |
| Current smoker | 18.5(260) | 22.6(487) | 0.003 | 13.4(943) | 15.8(1675) | <0.0001 | 5.3(176) | 7(313) | 0.001 |
| CABG | 2.6(36) | 1.9(41) | 0.2 | 10.6(748) | 16.9(1794) | <0.0001 | 12.3(412) | 20.5(909) | <0.0001 |
| Number of co-morbidities (±SD) | 1.8 ± 1.4 | 1.8 ± 1.3 | 0.4 | 3.0 ± 1.9 | 3.2 ± 1.9 | <0.0001 | 2.9 ± 1.8 | 3.3 ± (1.9 | <0.0001 |
| Cause of ESRD % (N) | 0.02 | <0.0001 | <0.0001 | ||||||
| Vascular | 6.9(97) | 9.3(200) | 17.2(1214) | 18.3(1938) | 39.3(1312) | 39.3(1744) | |||
| DM | 31.1(437) | 31.4(676) | 45.8(3227) | 44.3(4694) | 26.8(897) | 25(1111) | |||
| GN | 33.6(472) | 31.9(687) | 12.2(859) | 13.7(1449) | 8.7(291) | 8.8(389) | |||
| Obstruction | 4.6(64) | 3.9(83) | 2.2(153) | 3.0(314) | 2.4(81) | 4.0(179) | |||
| Interstitial | 1.6(22) | 0.6(13) | 1.3(94) | 0.7(74) | 1.6(53) | 0.9(38) | |||
| PCKD | 5.8(82) | 5.3(114) | 4.6(323) | 4.2(445) | 1.8(61) | 1.6(73) | |||
| Other | 7.8(109) | 8.2(177) | 8.6(607) | 7.9(841) | 6.5(217) | 6.3(279) | |||
| Unknown | 8.7(123) | 9.4(202) | 8.0(567) | 7.8(830) | 12.9(430) | 14.2(629) | |||
| Serum Albumin g/L (±SD) | 30.1 ± 10.5 | 31.5 ± 10.3 | <0.0001 | 31.1 ± 9.5 | 31.8 ± 9.3 | <0.0001 | 32.2 ± 8.8 | 32.2 ± 7.1 | 0.8 |
| Hemoglobin g/L (±SD) | 93.9 ± 26.7 | 97.1 ± 23.9 | <0.0001 | 99.5 ± 32.2 | 100.3 ± 21.1 | 0.03 | 102.0 ± 25.3 | 102.7 ± 23.9 | 0.2 |
| AVF/AVG % (N) | 11.6(163) | 17.3(372) | <0.0001 | 16.6(1168) | 20.6(2181) | <0.0001 | 14.5(483) | 18.9(838) | <0.0001 |
N number, % percentage, SD standard deviation, BMI body mass index, IQR interquartile range, km kilometer, Atlantic include Newfoundland, New Brunswick, Nova Scotia, prince Edward Island, Central Ontario, Prairie Manitoba, Saskatchewan, Alberta, Pacific British Columbia. CAD coronary artery disease, AMI acute myocardial infarction, DM diabetes mellitus, HTN hypertension, CABG coronary artery bypass graft, ESRD end stage renal disease, g/L grams per litre, Any pre-dialysis was defined as contact with a Nephrologist 30 days prior to renal replacement therapy initiation, AVF/AVG arteriovenous fistula/arteriovenous graft.
Association of sex, age and mortality
In both unadjusted and adjusted models, mortality for women was similar to men (HR 0.99 95% CI 0.96-1.03) however it varied by age groups (age X sex interaction p = 0.002). In individuals < 45 years of age, there were 317 (22.5%) and 391 (18.2%) deaths in women and men respectively, an absolute difference of 4.3% (crude mortality rate: women 4.53 and men 3.49 per 100 patient years, Table 2). After adjustment, this difference in mortality persisted as women under the age of 45 had a higher mortality compared to men (HR 1.31 95% CI 1.12-1.52) (see Figure 1). Conversely, women over 75 years of age experienced a modest survival benefit relative to men (age 75–85: HR 0.93 95% CI 0.87-0.99, age > 85: HR 0.82 95%CI 0.70-0.96).
Table 2.
Crude numbers and proportions of mortality on hemodialysis in women according to age categories with men as the referent
| Age group (in years) | Women | Male | ||
|---|---|---|---|---|
| Number of deaths | Proportion of total | Number of deaths | Proportion of total | |
| <45 | 317 | 22.5 | 391 | 18.2 |
| 45-55 | 499 | 35.0 | 833 | 35.2 |
| 55-65 | 1120 | 48.3 | 1667 | 46.6 |
| 65-75 | 1947 | 59.0 | 2753 | 59.3 |
| 75-85 | 1880 | 65.0 | 2568 | 67.3 |
| >85 | 297 | 66.0 | 438 | 70.2 |
Figure 1.
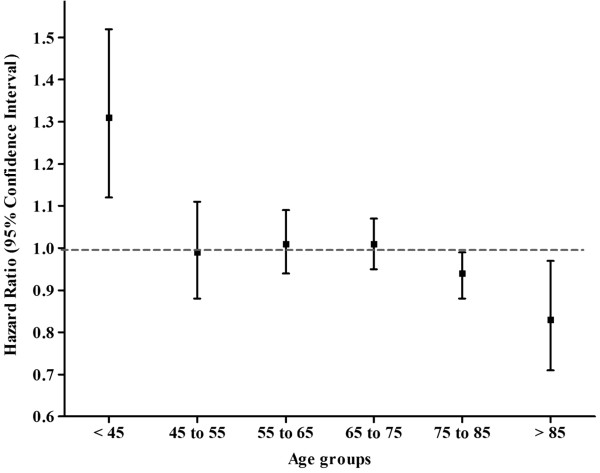
Adjusted survival in women on hemodialysis stratified by age with men as the referent. *Model adjusted for demographics, co-morbidities, body mass index (BMI), distance from centre, pre-dialysis care, cause of end stage renal disease, geographic region, serum hemoglobin and albumin.
Association of sex, age and mortality accounting for transplantation
In our analyses accounting for transplantation we used fewer age categories (<40, 40–50, 51–60, >60) to reflect that few individuals greater than 60 years of age receive a renal transplant. Utilizing a Fine and Gray model accounting for the competing risk (hazard ratio competing HRc) of transplantation, the hazard ratio for mortality in women compared to men was similar as traditional cox models (HR) (age < 40: HRc 1.32 (1.14-1.54) HR 1.35 (1.10-1.66), age 40–50 HRc 1.01 (0.90-1.13) HR 1.04 (0.90-1.20), age 51–60 HRc 1.03 (0.95-1.11) HR 1.01 (0.93-1.11), age > 60 HRc 1.02 (0.96-1.08) HR 1.00 (0.96-1.04). Women were less likely to receive a renal transplant compared to men, especially in those > 50 years of age (age 51–60: HRc 0.65 95% CI 0.55-0.77, > 60: HRc 0.56 95% CI 0.41-0.76).
Discussion
In this large national cohort of patients on chronic hemodialysis, substantial mortality differences were evident among the sexes based on age. Although overall mortality for men and women after comprehensive adjustment was similar, age was a significant effect modifier as women under the age of 45 years of age had a substantially higher mortality risk compared to men of a similar age. In contrast, women over the age of 75 years of age experienced a modestly lower mortality, an observation consistent with data in the general population. These differences persisted after accounting for the competing risk of transplantation and after comprehensive adjustment for confounders.
The finding that mortality is increased in women < 45 years of age on hemodialysis relative to men is congruent with finding from previous studies from Europe and the United States [2, 8, 9]. Crude mortality rates for hemodialysis patients from the United States Renal Data System (USRDS) show higher mortality in women under the age of 60 years of age compared to men with the largest disparity among those aged 20–29 years of age (Crude mortality rate male 4.93 women 6.29 per 100 patient years; relative rate 1.27). Similarly, data from the Renal Epidemiology and Information Network (REIN) registry in France reported an increase in the standardized mortality ratio in women relative to men, predominantly due to mortality in the first four years on dialysis. Our findings are consistent with these previous reports and demonstrate the mortality difference persists after adjustment for numerous confounders and after accounting for the competing outcome of transplantation.
We observed women greater than 75 years of age had a higher mortality compared to men. We found absolute mortality reductions of 2.3% and 4.2% in women 75 to 85 years of age and over 85 years of age relative to similar aged men. This observation mimics those seen in the Canadian general population where women experience, on average, an additional 4 years of longevity relative to men [13]. However it is unclear if the survival benefit is simply an extension of those observed in the general population or due to the possibility of a selection bias. There have been numerous reports that elderly women do not receive equal access to cardiovascular procedures, surgical interventions and kidney transplantation [14–16]. Elderly women may not choose or be offered chronic hemodialysis to the same extent as men thus altering the population on chronic hemodialysis. Further investigations regarding sex-based differences in acceptance and offer of dialysis treatment are required.
It is well known that kidney transplantation as a treatment for kidney failure is less frequent among women than men. In our cohort, we also found that the likelihood of receiving a kidney transplant was lower across all age categories in women. However, these differences do not explain the age-related mortality differences among the sexes since, after accounting for the competing risk of transplantation, there was little change in the observed mortality risk. Thus young women on dialysis are disadvantaged by both a higher risk of mortality and a reduced likelihood of receiving a renal transplant.
We did find several risk factors that may contribute to the higher mortality observed in younger woman on dialysis. Woman less than 45 years of age had an almost two-fold increase in malignancy, were more likely to have glomerulonephritis, obstruction or interstitial disease as their cause of renal failure, have a lower serum albumin and hemoglobin and were 5.7% less likely to initiate dialysis with a fistula. Of these only vascular access represents a potentially modifiable risk factor. Multiple studies have reported lower rates of AVF among women however it remains unclear if this represents actual differences in patient agreement for AVF, referral rates, AVF creation and/or maturation among the sexes [17–19]. Technical difficulties may include smaller vessel diameter or early thrombosis. Miller et al. reported post-AVF creation women are less likely to achieve fistula patency and are more likely to have early thrombosis and undergo more salvage procedures compared to men. A similar increase in complications and the need for more revascularization procedures has been reported in AVG in women compared to men [20]. It is possible that there may be important cosmetic considerations as young women may be less agreeable to AVF surgery. Attitudes towards vascular access suggest body image is an important consideration however whether it is sex-specific remains unclear [21].
The main strengths of our study are the use of a large national cohort with over 10 years of incident patient follow up. CORR captures data on almost all patients who initiate hemodialysis in Canada. We were able to perform more comprehensive covariate adjustment than previous studies including co-morbidities, modality, vascular access, laboratory parameters, geography, pre-dialysis care and distance to dialysis facility. We also accounted for the competing outcome of transplantation in our survival analysis.
Conversely, our study had limitations. As in any observational study, residual confounding cannot be ruled out as an alternative explanation for our findings. We lacked detailed information regarding the cause of death. We did not account for modality transitions namely initiation of dialysis on peritoneal and transitions to hemodialysis. Our vascular access measurements were taken at initiation of dialysis, did not reflect changes that occur over time and may not reflect access function. The distance from dialysis centre was direct linear distance and may not accurately reflect driving distances or travelling time. We lacked information on the source (living, deceased) of organ procurement.
Conclusion
In conclusion we found that age is an important effect modifier in survival of women on hemodialysis. Young women experienced a considerably higher mortality relative to men whereas older women appear to have a survival advantage. This was consistent after accounting for the competing risks of renal transplantation. Further sex-specific investigations are required into the mechanisms and possible prevention of these mortality disparities.
Methods
Study design and cohort development
This study was approved by the Research Board and the Hospital Ethics Board at St. Boniface Hospital in Winnipeg, Manitoba. We used the Canadian Organ Replacement Registry (CORR) as the source cohort for this analysis. CORR is a validated ESRD registry that captures data on all dialysis patients in Canada including demographics, death, dialysis modality, comorbidities and transplantation who supply written consent [22, 23]. We included all adult patients starting hemodialysis between January 1, 2001 and December 2009 who survived greater than 90 days. Patients with missing co-morbidity information were excluded (N =1,408). The final analytic cohort included 28,971 (Women 11,792 (40.7%), Men 17,179 (59.3%)). All data was de-identified, retrospective from the CORR and thus individual participant consent was not required.
Definitions
Co-morbid illnesses included a history of coronary artery disease, acute myocardial infarction, diabetes mellitus, pulmonary edema, peripheral vascular disease, malignancy, hypertension medication usage, current cigarette smoker, lung disease, any serious illness and stroke. Serious illness was defined as any illness that could shorten life expectancy to less than 5 years. Causes of ESRD included vascular disease, diabetes mellitus, glomerulonephritis, interstitial disease, polycystic kidney disease, obstruction, other and unknown. Provinces and territories were categorized as geographic regions as follows: Atlantic (New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland), Central (Ontario), Prairies (Alberta, Saskatchewan, Manitoba, Nunavut, Northwest Territories), Pacific (British Columbia, Yukon). Any pre-dialysis care was defined as contact with a Nephrologist for 30 or more days prior to hemodialysis initiation. Distance to centre was calculated as the direct linear distance in kilometres between a patients postal code from their primary residence at dialysis initiation to the nearest dialysis provider. Comorbidities and laboratory data were ascertained at the onset of ESRD. The outcome of interest was all-cause mortality and follow-up for outcomes was until December 31st, 2009.
Statistical analyses
Continuous variables of interest were summarized as mean or medians with standard deviation or inter-quartile range as appropriate. Differences in baseline characteristics were determined by student’s t-test for continuous variables and chi-square or the Mann–Whitney test for dichotomous variables.
To assess the relationship between the sexes and mortality, we examined both traditional cause-specific cox proportional hazards model and the modified risks regression model of Fine and Gray to account for the competing risk of transplantation [24]. Models were adjusted for demographics, co-morbidities, body mass index (BMI), distance from centre, pre-dialysis care, cause of ESRD, geographic region, serum hemoglobin and albumin. The competing risk accounted for in the Fine and Gray models was renal transplantation.
To assess whether age was an effect modifier for mortality among the sexes, an age X sex interaction term was added separately to the traditional cox and competing risks models. In separate models, age was entered both as a continuous variable and categorical variable (age < 45, 45–55, 55–65, 65–75, 75–85, 85+ years). Categories were created based on the number of death events and individual models were created per age stratum with men as the referent. As there are few kidney transplants with increasing age, age categories were collapsed in the competing risk models.
Multiple imputation was employed for missing values with a random draw from the predictive distribution from an imputation model repeated ten times [25]. The proportion and covariates with missing data that was imputed included BMI (8.2%), distance from centre (2.0%), cause of ESRD (2.9%), geographic region (0.2%), serum hemoglobin (15.4%) and albumin (21.5%). An iterative Markov chain Monte Carlo (MCMC) method was used and pooled estimates of 10 rounds of imputation reported. Imputed and non-imputed models were compared and as there were no substantive changes in point estimates, the pooled imputed results of 10 rounds of imputation were reported. Analyses were performed using PASW Version 18 and the Fine and Gray analyses were performed using R. All hypothesis tests were two sided with statistical significance to find as having a P value of <0.05.
Acknowledgement
MMS is provided salary support by the Jindal Research Chair for the Prevention of Kidney Disease at the University of Ottawa. NT is supported by the KRESCENT New Investigator Award, a joint initiative of the Kidney Foundation of Canada, Canadian Institute of Health Research and the Canadian Society of Nephrology.
Footnotes
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
MMS, NT contributed to the study design and conception. MMS and NT drafted the manuscript which was critically revised by all authors. MMS, NT, PK, CR, JM contributed to the analysis and interpretation of the data. JM was responsible for the statistical analyses. All authors have approved and of these final version of the manuscript.
Contributor Information
Manish M Sood, Email: msood99@gmail.com.
Claudio Rigatto, Email: CRIGATTO@sbgh.mb.ca.
Paul Komenda, Email: paulkomenda@yahoo.com.
Julie Mojica, Email: JMojica@exchange.hsc.mb.ca.
Navdeep Tangri, Email: ntangri@sogh.mb.ca.
References
- 1.ERA-EDTA Registry . ERA-EDTA Registry Annual Report 2010. Amsterdam: Department of Medical Informatics; 2012. [Google Scholar]
- 2.United States Renal Data System . USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 3.Segev DL, Kucirka LM, Oberai PC, Parekh RS, Boulware LE, Powe NR. Age and comorbidities are effect modifiers of gender disparities in renal transplantation. J Am Soc Nephrol. 2009;20(3):621–628. doi: 10.1681/ASN.2008060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloembergen WE, Port FK, Mauger EA, Wolfe RA. Causes of death in dialysis patients: racial and gender differences. J Am Soc Nephrol. 1994;5(5):1231–1242. doi: 10.1681/ASN.V551231. [DOI] [PubMed] [Google Scholar]
- 5.Sehgal AR. Impact of quality improvement efforts on race and sex disparities in hemodialysis. JAMA. 2003;289(8):996–000. doi: 10.1001/jama.289.8.996. [DOI] [PubMed] [Google Scholar]
- 6.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 7.Allon M, Ornt DB, Schwab SJ, Rasmussen C, Delmez JA, Greene T. Factors associated with the prevalence of arteriovenous fistulas in hemodialysis patients in the HEMO Study. Kidney Int. 2000;58(5):2178–85. doi: 10.1111/j.1523-1755.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 8.Villar E, Remontet L, Labeeuw M, Ecochard R. Effect of age, gender, and diabetes on excess death in end-stage renal failure. J Am Soc Nephrol. 2007;18(7):2125–2134. doi: 10.1681/ASN.2006091048. [DOI] [PubMed] [Google Scholar]
- 9.Carrero JJ, De Jager DJ, Verduijn M, Ravani P, De Meester J, Heaf JG. Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol. 2011;6(7):1722–1730. doi: 10.2215/CJN.11331210. [DOI] [PubMed] [Google Scholar]
- 10.Kitty J, Jager KJ, Stel VS, Zoccali C, Wanner C, Dekker FW. The issue of studying the effect of interventions in renal replacement therapy — to what extent may we be deceived by selection and competing risk? Nephrol Dial Transplant. 2010;25(12):3836–3839. doi: 10.1093/ndt/gfq540. [DOI] [PubMed] [Google Scholar]
- 11.Marion Verduijn DCG, Friedo WD, Kitty JJ, Saskia le C. The analysis of competing events like cause-specific mortality—beware of the Kaplan–Meier method. Nephrol Dial Transplant. 2010;26(1):56–61. doi: 10.1093/ndt/gfq661. [DOI] [PubMed] [Google Scholar]
- 12.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306(6):620–626. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Statistics Canada: Life Expectancy at Birth, by Sex, by Province. Ottawa, Ontario: Statistics Canada catalogue; accessed March 13,2014
- 14.Melberg T, Kindervaag B, Rosland J. Gender-specific ambulance priority and delays to primary percutaneous coronary intervention: a consequence of the patients’ presentation or the management at the emergency medical communications center? Am Heart J. 2013;166(5):839–845. doi: 10.1016/j.ahj.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen JT, Berger AK, Duval S, Luepker RV. Gender disparity in cardiac procedures and medication use for acute myocardial infarction. Am Heart J. 2008;155(5):862–868. doi: 10.1016/j.ahj.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller LM, Sood MM, Sood AR, Reslerova M, Komenda P, Rigatto C, Bueti J. Cardiovascular disease in end-stage renal disease: the challenge of assessing and managing cardiac disease in dialysis patients. Int Urol Nephrol. 2010;42(4):1007–1014. doi: 10.1007/s11255-010-9857-x. [DOI] [PubMed] [Google Scholar]
- 17.Astor BC, Eustace JA, Powe NR, Klag MJ, Fink NE, Coresh J. Type of vascular access and survival among incident hemodialysis patients: the choices for healthy outcomes in caring for esrd (choice) study. J Am Soc Nephrol. 2005;16(5):1449–1455. doi: 10.1681/ASN.2004090748. [DOI] [PubMed] [Google Scholar]
- 18.Miller C, Robbin ML, Allon M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int. 2003;63(1):346–352. doi: 10.1046/j.1523-1755.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 19.Reddan D, Klassen P, Frankenfield DL, Szczech L, Schwab S, Coladonato J. National profile of practice patterns for hemodialysis vascular access in the united states. J Am Soc Nephrol. 2002;13(8):2117–2124. doi: 10.1097/01.ASN.0000022422.79790.A8. [DOI] [PubMed] [Google Scholar]
- 20.Astor BC, Coresh J, Powe NR, Eustace JA, Klag MJ. Relation between gender and vascular access complications in hemodialysis patients. Am J Kidney Dis. 2000;36(6):1126–1134. doi: 10.1053/ajkd.2000.19816. [DOI] [PubMed] [Google Scholar]
- 21.Xi W, Harwood L, Diamant MJ, Brown JB, Gallo K, Sontrop JM. Patient attitudes towards the arteriovenous fistula: a qualitative study on vascular access decision making. Nephrol Dial Transplant. 2011;26(10):3302–3308. doi: 10.1093/ndt/gfr055. [DOI] [PubMed] [Google Scholar]
- 22.Moist LM, Richards HA, Miskulin D, Lok CE, Yeates K, Garg AX. A validation study of the canadian organ replacement register. Clin J Am Soc Nephrol. 2011;6(4):813–818. doi: 10.2215/CJN.06680810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canadian Institute for Health Information, Canadian Organ Replacement Register Annual Report . Treatment of End-Stage Organ Failure in Canada, 2001 to 2010. Ottawa, Ont: CIHI; 2011. [Google Scholar]
- 24.Jason PF, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 25.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. doi: 10.1191/096228099671525676. [DOI] [PubMed] [Google Scholar]


