Figure 4.
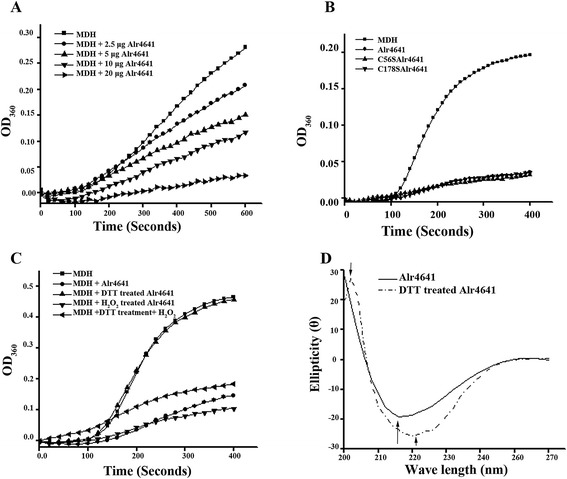
Alr4641 functions as a molecular chaperone. (A) Chaperone activity. Light scattering due to thermal aggregation of malate dehydrogenase (MDH, 5 μM) in the presence of different concentrations of Alr4641 (as indicated in the figure) was monitored with a spectrophotometer at 360 nm. (B) Light scattering of MDH was monitored (as described in A) in the presence of Alr4641C56S or Alr4641C178S or Alr4641 (20 μg each). (C) Chaperone activity of oxidized or reduced Alr4641. The purified Alr4641 protein was treated with H2O2 (10 mM) or DTT (10 mM) for 60 min and tested for chaperone activity with MDH (5 μM). In another reaction, the DTT-treated Alr4641 was incubated with H2O2 (5 mM) for 30 min and then employed for the chaperone assay. (D) Secondary structure analysis. The purified Alr4641 protein treated with DTT (10 mM) for 30 min or the control Alr4641 protein i.e. without DTT treatment (as indicated in the figure), was analyzed in a CD spectropolarimeter.
