Figure 9.
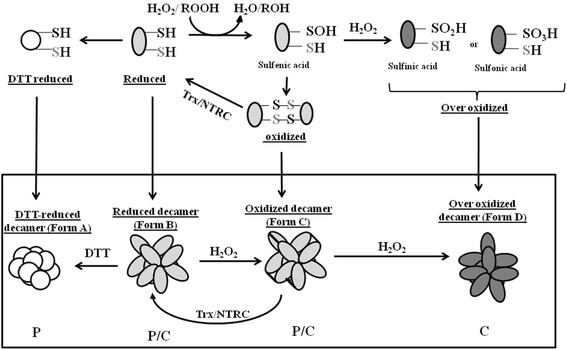
A model depicting redox-dependent functional switching of Alr4641. Rectangular box in the lower panel represents the oligomeric (decameric) structure of Alr4641 while the upper panel shows the state of catalytic cysteine residues of the individual monomeric units. Various conformational forms of the Alr4641 protein i.e. DTT-reduced decamer (Form-A), reduced decamer (Form-B), oxidized decamer (Form-C) and over-oxidized decamer (Form-D) are shown in the figure. Alr4641 exists as decamer irrespective of its redox state or disulphide linkage status) in all the above-mentioned conformations. Monomeric units corresponding to various forms are depicted as follows: Form-A, white circle; Form-B and Form-C, light-grey oval and Form-D, dark-gray oval. Form-B (with free thiol groups) on oxidation with H2O2 forms inter-molecular disulfide bonds (indicated with black lines in the lower panel), resulting in the formation of oxidized decamer (i.e. Form-C). Form-B is regenerated from Form-C by electron donors like Trx and NTRC. However, with excess H2O2, the cysteinyl residue of the peroxidatic cysteine of Form-B is over-oxidised to sulfinic/sulfonic acid (Form-D). Reduction of Alr4641 with DTT not only results in the loss of disulfide bonds but also changes the overall structure of the protein (Form-A). Form-B, Form-C and Form-D show chaperone activity (indicated by “C”), but Form-A fails to show this activity. On the other hand, Form-A, Form-B and Form-C function as peroxidase (indicated by “P”), whereas the over-oxidized Alr4641 i.e. Form-D is unable to do so. To summarize, under reducing conditions, Alr4641 is more likely to function as a peroxidase, whereas under oxidizing surroundings, it is more likely to work as a chaperone.
