Figure 1.
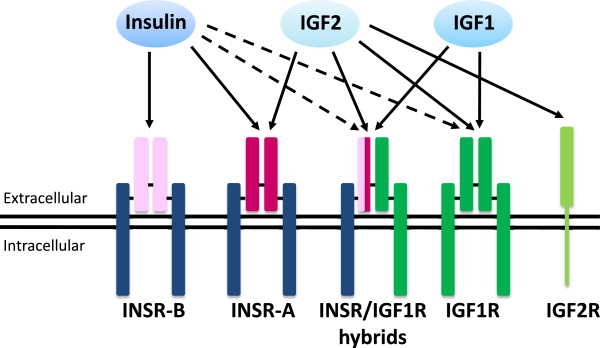
Receptors for insulin, IGF1 and IGF2. INSR and IGF1R are composed of two αβ dimers which associate to form heterotetrameric complexes. The αβ dimers are linked together by disulfide bonds and two dimers are also linked by disulfide bonds to form the tetramer. The α subunit is the extracellular portion of the receptor while the β subunit spans the membrane and its cytoplasmic portion interacts with IRS proteins which are key intracellular mediators of insulin/IGF signaling. Single αβ dimers are derived from separate genes and the INSR has two splice variants, INSR-B and INSR-A. Each variant shares the same membrane-spanning β subunit (dark blue) but differs in the extracellular α subunit (light pink or dark pink, respectively). The IGF1R has different α and β subunits compared to the INSR (dark green). These combinations of αβ dimers allow for hybrid receptors, which bind insulin, IGF1, and IGF2 with differing affinities. The schematic shows the αβ dimers, α2β2 hybrid receptors, and the known ligands that bind each receptor. Relative binding affinities are represented by arrows, where a solid arrow signifies a higher binding affinity than a broken arrow.
