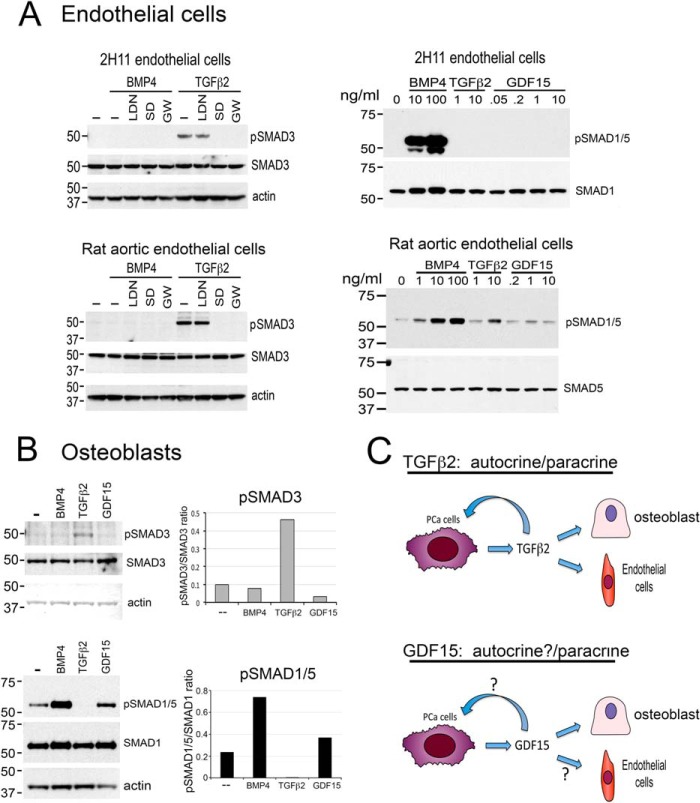
Fig. 3.
TGFβ2 and GDF15 exert paracrine effects by stimulating SMAD phosphorylation in endothelial cells and osteoblasts. A, Left panel, TGFβ2 stimulates SMAD3 phosphorylation in 2H11 endothelial cells and rat aortic endothelial cells. The signaling is inhibited by SD208 and GW788388, two TGFβ receptor 1 inhibitors, but not by LDN193189, an inhibitor of BMP type 1 receptors ALK2 and ALK3. Right panel, TGFβ2 stimulates SMAD1/5 phosphorylation in a dose-dependent manner in rat aortic endothelial cells, but not in 2H11 cells. GDF15 did not affect SMAD1/5 phosphorylation in both 2H11 and rat aortic endothelial cells. B, TGFβ2 stimulates SMAD3, but not SMAD1/5, phosphorylation in osteoblasts. GDF15 stimulates SMAD1/5, but not SMAD3, phosphorylation in osteoblasts. C, Role of TGFβ2 and GDF15 in PCa-118b tumor. Based on receptor expression and SMAD signaling, TGFβ2 functions as an autocrine as well as a paracrine factor in PCa-118b tumor. GDF15 can function as a paracrine factor on osteoblasts. Whether GDF15 can function as an autocrine factor is as yet unclear.