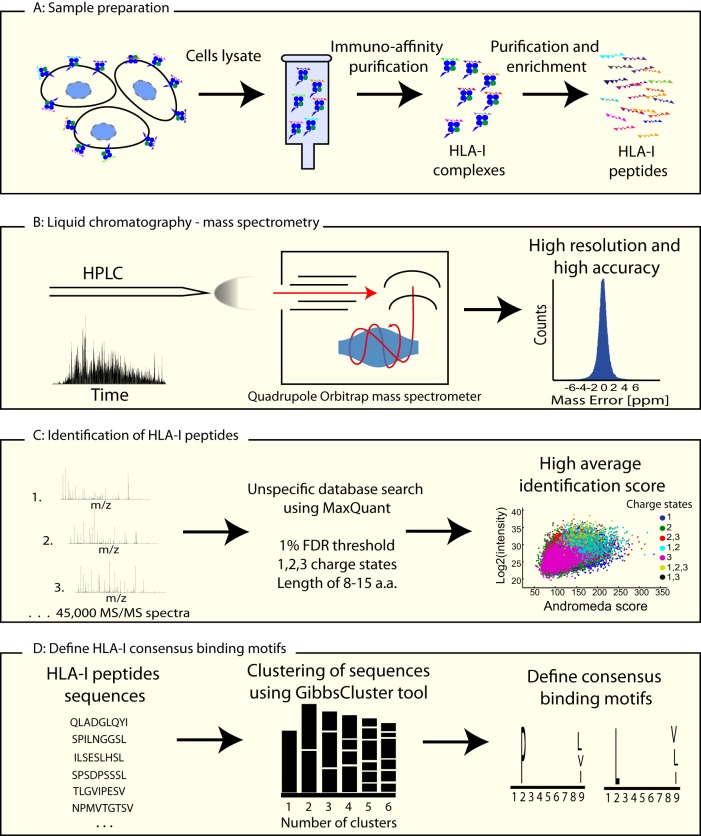
Fig. 1.
Schematic overview of HLA-I peptidomics. A, Sample preparation. HLA-I complexes were immunoaffinity purified from cells lysates using anti-HLA-I (W6–32) antibody cross-linked to Protein-A Sepharose beads. HLA-I peptides were purified from the heavy chain based on their hydrophobicity using a C-18 column. B, Liquid chromatography and mass spectrometry. The enriched mixtures of HLA-I peptides were measured on a quadrupole Orbitrap mass spectrometer (Q Exactive), resulting in high resolution and high mass accuracy at the MS and MS/MS levels. C, Identification of HLA-I peptides. HLA-I peptides were analyzed with MaxQuant software, using an unspecific search, allowing the identification of peptides with one, two, and three charge states. In total, 22,244 unique HLA-I peptides were identified with a median identification score of 123 using a threshold of 1% FDR at the peptide level. D, HLA-I consensus binding motifs. Using the GibbsCluster tool the consensus binding motifs were defined from the identified peptides sequences.