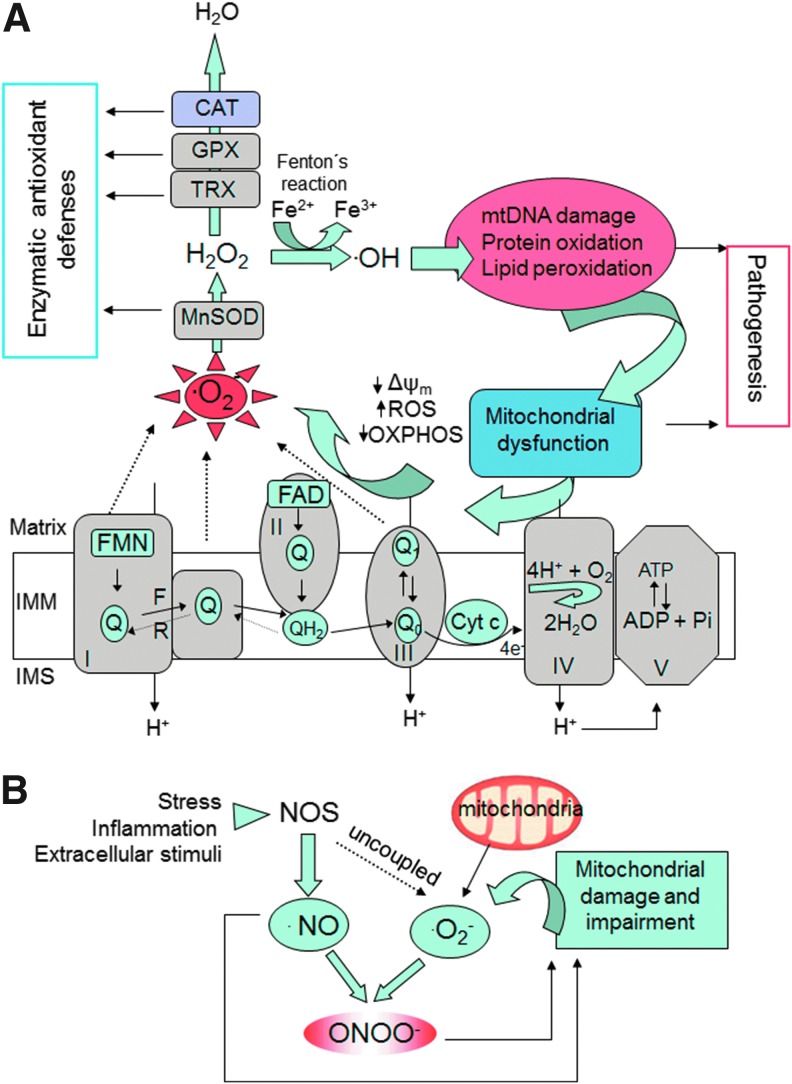
FIG. 2.
Mechanisms of oxidative stress involving mitochondria. (A) The mitochondrial ETC reoxidizes reduced cofactors (NADH and FADH2) using molecular oxygen as the final electron acceptor, and the energy released in this process is captured in the form of ATP. Several sites of the ETC (CI and CIII and the reverse electron flow at Complex II) generate O2•−. This radical is further converted into H2O2 by mitochondrial SOD. Other antioxidant enzymes within mitochondria involve TRX and GPX, and in certain tissues (liver, cardiac muscle) also CAT. Through the Fenton reaction, H2O2 is converted into •OH, a molecule that produces oxidative cell injury through DNA damage, carboxylation of proteins, and lipid peroxidation. Damaged mitochondria are dysfunctional and further produce free radicals, thus generating a “vicious cycle.” (B) Mechanisms of nitrosative stress. •NO is produced by the activity of intracellular NOS. •NO can be combined with O2•− to produce ONOO−, a molecule that acts as a strong oxidant and can damage many cellular structures and alter their function. Reactive nitrogen species such as ONOO− contribute to further mitochondrial dysfunction. •NO, nitric oxide; •OH, hydroxyl radical; CAT, catalase; ETC, electron transport chain; F, forward; GPX, glutathione peroxidase; H2O2, hydrogen peroxide; IMM, inner mitochondrial membrane; IMS, intermembrane space; NOS, nitric oxide synthase; O2•−, superoxide anion; ONOO−, peroxynitrite; OXPHOS, oxidative phosphorylation; R, reverse; SOD, superoxide dismutase; TRX, thioredoxin; Δψm, mitochondrial membrane potential. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars