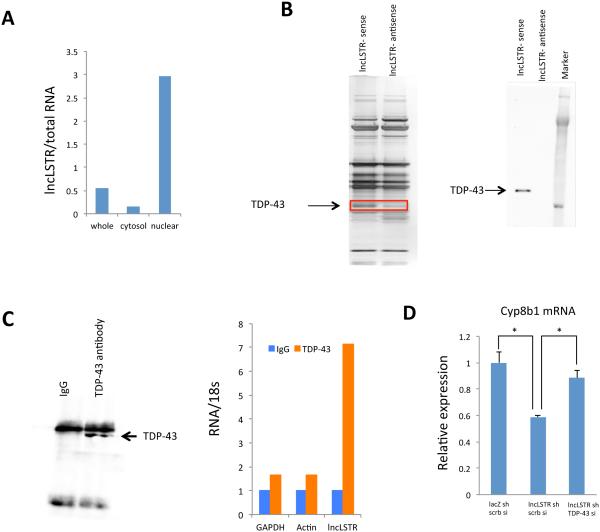
Figure 5. LncLSTR interacts with TDP-43.
(A) Levels of lncLSTR in whole cell, cytosolic or nuclear fractions of liver tissues pooled from 4 mice.
(B) Left, silver stained SDS-PAGE gel analysis of proteins in nuclear extract of liver tissues that are bound to biotinylated lncLSTR or its antisense. The highlighted regions were analyzed by mass spectrometry, identifying TDP-43 as a protein unique to lncLSTR. Right, immunoblotting analysis of proteins in nuclear extract of liver tissues that are bound to biotinylated lncLSTR or its antisense using an anti-TDP-43 antibody.
(C) Left, anti-TDP-43 immunoblotting analysis of proteins in immunoprecipitates of liver tissues using an anti-TDP-43 antibody. Right, Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), actin and lncLSTR RNA levels in immunoprecipitates of liver tissues using an anti-TDP-43 antibody.
(D) Cyp8b1 expression in primary hepatocytes receiving lacZ shRNA, lncLSTR shRNA, scramble siRNA (scrb si) or TDP-43 siRNA in combination as indicated (the Ct levels of Cyp8b1 and TDP-43 are ~27 and ~23 respectively).
Error bars represent SEM, *p<0.05.