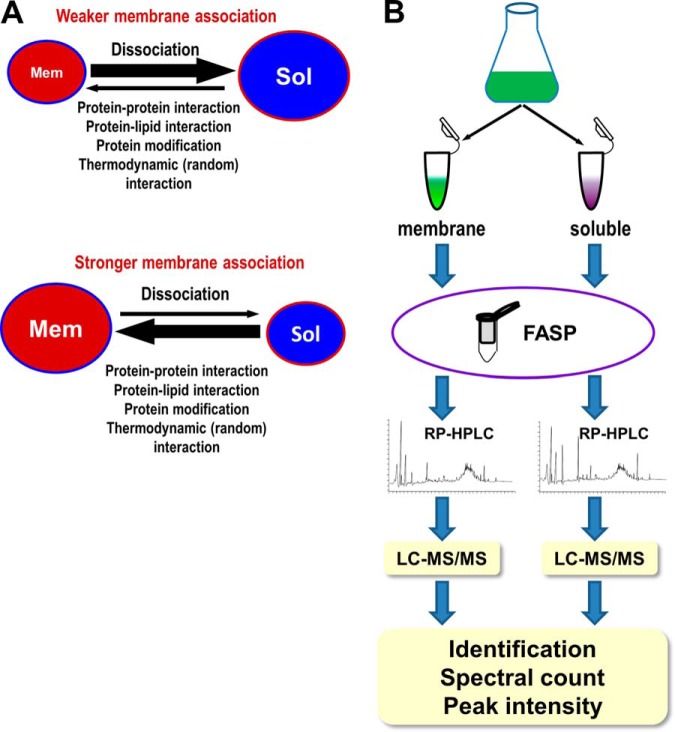
Fig. 1.
Rational and experimental design. A, Schematic representation of the rational for measuring the relative tightness of membrane association for non-TM containing proteins. Non-TM containing proteins can dynamically associate with and dissociate from the membranes. Fast dissociation and slow association for a protein indicate a weak membrane association and big soluble pools (Sol) for such a protein (upper panel), whereas slow dissociation and fast association for a protein indicate a strong membrane association and big membrane pool (Mem) for such a protein (lower panel). The sizes of the pools, which reflect the relative tightness of membrane association, can be measured at the proteome scale by measuring the relative protein abundance using peptide spectral counts and peak intensities. B, Schematic representation of the experiment design. The prefractionation of sample peptides was performed by basic RP-HPLC, and the fractionated peptides were further separated by online acidic RP-HPLC and analyzed by high resolution tandem MS (LC-MS/MS).