Fig. 7.
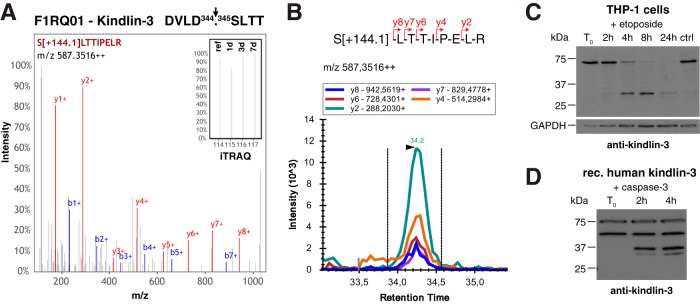
Processing of kindlin-3 in wound exudates. A, Cleavage site and representative spectrum of generated neo-N-terminal peptide of kindlin-3. Inset shows a close up of iTRAQ reporter ions. B, SRM data for kindlin-3 neo-N terminus in wound exudates. Five transitions of fragment ions of the iTRAQ[+144.1]-labeled precursor peptide were monitored. C, Cleavage of kindlin-3 in THP1 monocytes upon induction of apoptosis. THP1 cells were treated with etoposide and analyzed for processing of kindlin-3 by immunoblot using an antibody specific to human kindlin-3 (49). D, Direct processing of kindlin-3 by caspase-3. Recombinant human kindlin-3 was incubated with recombinant active caspase-3 and monitored for processing by immunoblot analysis. A second band at 50 kDa was observed in the commercial kindlin-3 preparation irrespective of incubation with caspase-3, whereas lower molecular weight fragments only appeared upon treatment with the protease.
