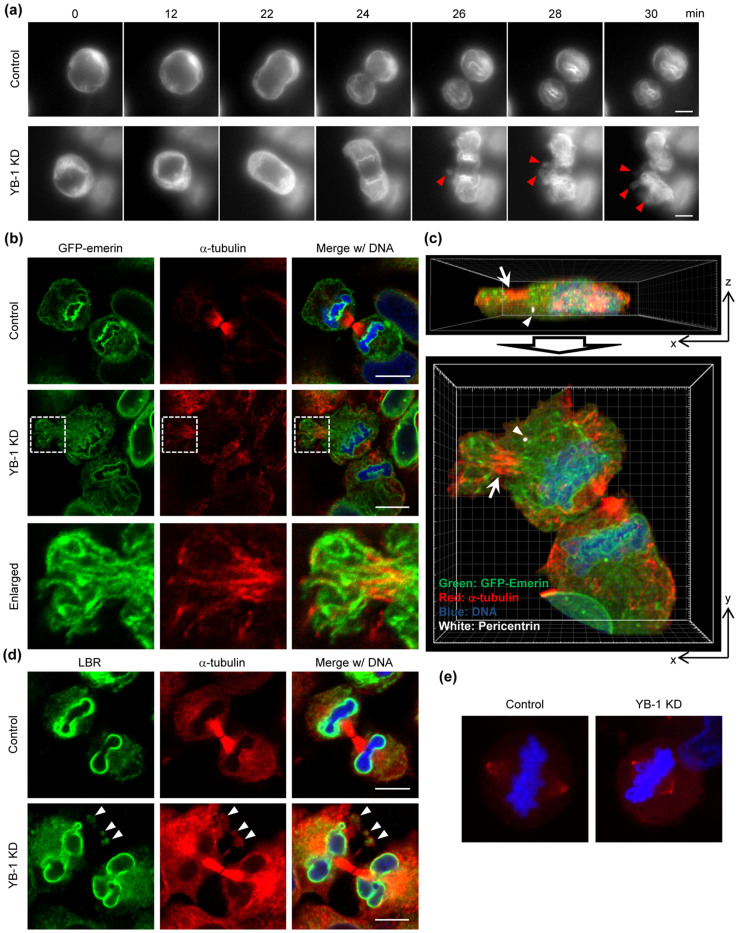
Figure 3. Defect in NE reassembly at telophase in YB-1 KD cells.
(A, B, C) At 48 h post transfection with either non-targeting (control) or YB-1 siRNA (YB-1 KD), HeLa-GFP-emerin cells were subjected to the live cell imaging using AxioCam MRm camera (Carl Zeiss; Fig. 3A) and the indirect immunofluorescence assays with anti-α-tubulin (Fig. 3B, C) and anti-pericentrin (Fig. 3C) antibodies. For live-cell imaging, images were acquired at 2-min intervals for 90 min (see also Supplementary Movie S3 and S4), and the sequential images of each movie are shown in Fig. 3A. In Fig. 3B, areas in white boxes are enlarged. Fig. 3C represents the projection images of xz- (upper panel) and xy-sections (lower panel) of YB-1 KD cells shown in Fig. 3B. Image processing was performed using IMARIS 7.2 software (Carl Zeiss). The cells adjacent to the telophase cell were omitted from images. Arrowheads and arrows indicate the centrosome (white) and non-centrosomal microtubules (red), respectively. Nuclei were counter-stained with DAPI (blue). Scale bar, 10 μm. (D) At 48 h post transfection with either non-targeting (control) or YB-1 siRNA (YB-1 KD), HeLa cells were subjected to the indirect immunofluorescence assay with anti-LBR (green) and anti-α-tubulin (red) antibodies. Nuclei were counter-stained with DAPI (blue). The spore-like structures are indicated by arrowheads. Scale bar, 10 μm. (E) At 48 h post transfection with either non-targeting (control) or YB-1 siRNA (YB-1 KD), HeLa cells were subjected to the indirect immunofluorescence assay with anti-γ-tubulin antibody (red). Nuclei were counter-stained with DAPI (blue).