Abstract
Adaptor protein-3 (AP-3) is a heterotetrameric complex, which regulates vesicular trafficking. Mutations of the β3A subunit cause the Hermansky–Pudlak syndrome type 2 (HPS-2), a rare genetic disease characterized by albinism, platelet defects, and recurrent infections. Likewise, pearl mice, which lack functional AP-3, show several HPS-2 defects. The AP-3 absence results in defective toll-like receptor trafficking and signaling in dendritic cells (DC), but its effect on the efficiency of the in vivo antiviral response is unclear. We evaluated the impact of AP-3 deficiency on the distribution of DC subsets, interferon (IFN) production, and the susceptibility to murine cytomegalovirus (MCMV) infection. Pearl mice showed a distribution and frequency of conventional (cDC) and plasmacytoid DC (pDC) similar to that of wild-type mice both before and after MCMV infection. Moreover, pearl mice controlled MCMV infection even at high virus doses and showed a normal production of IFN-α. Since pDC, but not cDC, from pearl mice showed an impaired IFN-α and tumor necrosis factor-α production in response to prototypic DNA (MCMV and Herpes Simplex virus) or RNA (Vesicular Stomatitis virus) viruses in vitro, it is likely that MCMV infection can be controlled in vivo independently of an efficient production of IFN-α by pDC, and that the AP-3 complex has a minimal impact on protective antiviral responses.
Adaptor complexes [adaptor protein (AP)-1 through 5] are heterotetrameric proteins involved in distinct intracellular vesicular transport pathways. AP-3 escorts proteins from the early trans-Golgi network to lysosome-related organelles (Feng and others 1999; Dell'Angelica 2009). A mutation in the gene ADTB3A, which encodes for the β3A subunit, causes the Hermansky–Pudlak syndrome type 2 (HPS-2), an autosomal recessive inherited disease characterized by partial albinism, prolonged bleeding, and immunodeficiency (Badolato and Parolini 2007).
Interestingly, human HPS-2 has its murine counterpart in the pearl strain of mice, which bears a mutation in the AP3B1 gene, the mouse orthologous of ADTB3A, and shares many aspects of the human disease (Feng and others 1999).
Being responsible for protein trafficking to lysosomes, AP-3 affects important immune processes. In fact, both AP-3-deficient mice and HPS-2 patients show abnormalities involving the polarization of lytic granules inside cytotoxic T cells and the intracellular localization of CD1b and CD1d, which are required for the presentation of lipid antigens and NKT cell development (Elewaut and others 2003). AP-3 has been linked to toll-like receptor (TLR) signaling in dendritic cells (DC) and is required for TLR4 recruitment to phagosome and MHCII presentation of antigens internalized by phagocytosis (Mantegazza and others 2012). Furthermore, AP-3, as well as the other HPS proteins, is required for plasmacytoid DC (pDC) signaling through TLR7 and TLR9 (Blasius and others 2010; Sasai and others 2010). These facts point out the involvement of AP-3 in the development of efficient innate and adaptive immune responses. DC play a pivotal role in the response to a wide variety of viruses both in humans and mice, including cytomegalovirus (CMV) (Rölle and Olweus 2009; Rahman and others 2011). The experimental model of murine CMV (MCMV) infection greatly advanced our understanding of the DC role and, due to the many features shared with the species-specific human counterpart virus, it is widely used to investigate the innate and adaptive immune responses to CMV in vivo (Riera and others 2000; Krmpotic and others 2003). In this regard, it has been established that conventional DC (cDC) support productive MCMV infection both in vitro and in vivo (Andrews and others 2001), but they also have an important role in the control and clearance of viral infection. After infection, CD11b DC secrete innate immunity cytokines, including IFN-α, and are involved in NK cell activation. pDC are considered the main producers of IFN-α and IFN-β in response to MCMV infection in a TLR9- and MyD88-dependent manner (Krug and others 2004). However, several studies showed that, in addition to pDC, other cell types, such as cDC, macrophages, and lymphoid-tissue stromal cells, can produce IFN-α in response to Herpes Simplex virus (HSV) or CMV infections. Moreover, IFN-α production by non-pDC is largely TLR9 independent and responsible for most of the IFN-α produced (Hochrein and others 2004; Sozzani and others 2010). Although the AP-3 protein is required for TLR9 and TLR7 activation in pDC, it is unclear whether the pearl mice defect can influence the in vivo immune response to viral infections. The purpose of this study was to determine the degree of susceptibility of pearl mice to MCMV infection and to assess whether MCMV infection modulates DC subsets and IFN production in these mice.
To characterize DC, CD34+ bone marrow-derived cells from pearl mice (kindly provided by Prof. Dr. Stephan Ehl) and C57BL/6J wild-type (wt) mice (Charles River Breeding Laboratories) were purified by positive immunoselection and cultured with murine granulocyte macrophage colony-stimulating factor (mGM-CSF) (40 ng/mL) and Flt-3L (100 ng/mL) to generate cDC, or with Flt3L (200 ng/mL) only to generate pDC (Del Prete and others 2007). As previously shown (Sasai and others 2010), pearl mice DC were comparable to wt DC in terms of surface expression of CD11c, CD11b, MHC I and II, and CD80 (data not shown). Then, cDC and pDC from pearl mice were functionally characterized for cytokine production in response to different virus infections. Since TLR7 and TLR9 are required for pDC to sense viral nucleic acids, we investigated the response of AP-3-deficient pDC to prototypic DNA viruses, such as MCMV (Smith strain, ATCC VR.194) and HSV-2 (a clinical isolate), which stimulate pDC mainly through TLR9, and to a RNA virus, such as the Vesicular Stomatitis virus (serotype Indiana), which triggers IFN-α production by pDC mainly through TLR7 (Ahmed and others 2009). To this end, pDC were infected with the different viruses at a multiplicity of infection (MOI) of 1, or treated as a positive control for TLR-9 stimulation, with CpG 2216 type-A (20 μg/mL) for 24 h.
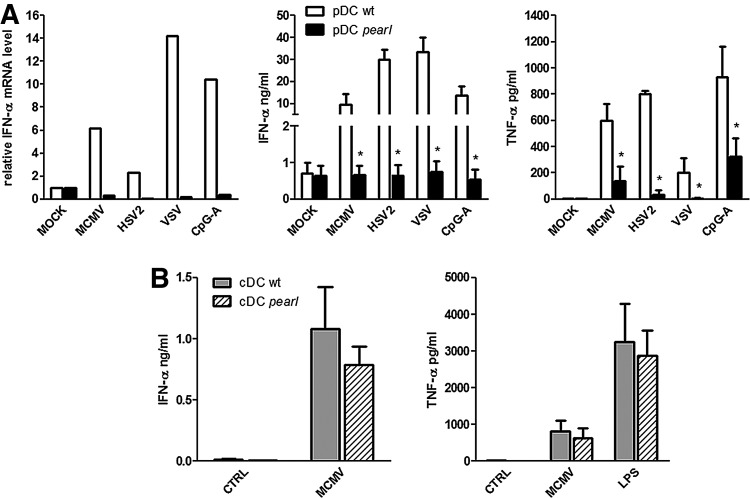
As shown in Fig. 1A, regardless of the virus, a severe defect in IFN-α production, both at the mRNA (left panel) and protein levels (middle panel), was observed in pearl pDC compared to wt pDC. A similar defect was observed in tumor necrosis factor-α (TNF-α) secretion as well (Fig. 1A, right panel). In contrast, no significant differences in IFN-α levels were observed after MCMV stimulation of pearl cDC compared to wt cDC (Fig. 1B, left panel), and cDC from pearl mice were able to produce normal amounts of TNF-α after either MCMV or lipopolysaccharide (LPS) stimulation (1 μg/mL) (Fig. 1B, right panel). Results reported in Fig. 1 indicate that the AP-3 deficiency in pearl mice selectively impairs the ability of pDC, but not of cDC, to respond to different viruses that signal through TLR7 or TLR9.
FIG. 1.
Bone marrow (BM)-derived plasmacytoid dendritic cells (pDC) from pearl mice are defective for interferon-α (IFN-α) production in response to virus infections. CD34+ BM-derived cells from pearl and C57B6/L wild-type (wt) mice were purified by positive immunoselection and treated to generate conventional DC (cDC) or pDC, as previously described (Del Prete and others 2004). pDC were infected at multiplicity of infection (MOI) of 1 with murine cytomegalovirus (MCMV), Herpes Simplex virus (HSV)-2, Vesicular Stomatitis virus (VSV) or treated with CpG ODN 2216 (CpG-A) (20 μg/mL). [(A), left panel] After 24 h, total RNA was extracted, retrotranscribed with the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc.) and real-time polymerase chain reaction performed with iQ™ SYBR Green Supermix (Bio-Rad Laboratories, Inc.) was carried out using primers specific for IFN-α (sense, 5′-CCACAGGATCACTGTGTACCTGAGA-3′; antisense, 5′-CTGATCACCTCCC AGGCACAG-3′). The cDNA was amplified for 45 cycles (95°C for 10 s and 60°C for 30 s). Results were normalized to the 18S signals and are presented as fold increase relative to the mRNA levels in unstimulated cells (assumed as the 1.0 value) (1 representative experiment out of 3). [(A), center and right panels] IFN-α and tumor necrosis factor-α (TNF-α) concentrations were measured by VeriKine Mouse IFN-α ELISA (PBL Interferon Source) and Duoset ELISA (R&D System) in the supernatants from BM-derived pDC stimulated as described above. (B) IFN-α and TNF-α concentrations were measured in the supernatants from BM-derived cDC infected with MCMV (MOI 1) or treated with lipopolysaccharide (LPS) (1 μg/mL) for 24 h. Data are expressed as mean±standard error of the mean (SEM) (n=3). Results were analyzed by the 2-tailed Student's t-test. *P<0.05.
Moreover, these observations suggest that AP-3 deficiency abolishes the TLR9-mediated IFN-α response of pDC during MCMV infection. This, in turn, may lead to an increased susceptibility to MCMV in vivo. In this regard, MCMV infection is considered a suitable experimental model to study systemic Herpesvirus infection, as well as the TLR-dependent and -independent mechanisms of type I IFN induction. In fact, in the host, MCMV spreads through the blood reaching the liver and spleen, which are the principal sites of viral replication, and induces a type I IFN response mediated by different cell-type-specific mechanisms of viral recognition and IFN expression (Steinberg and others 2009).
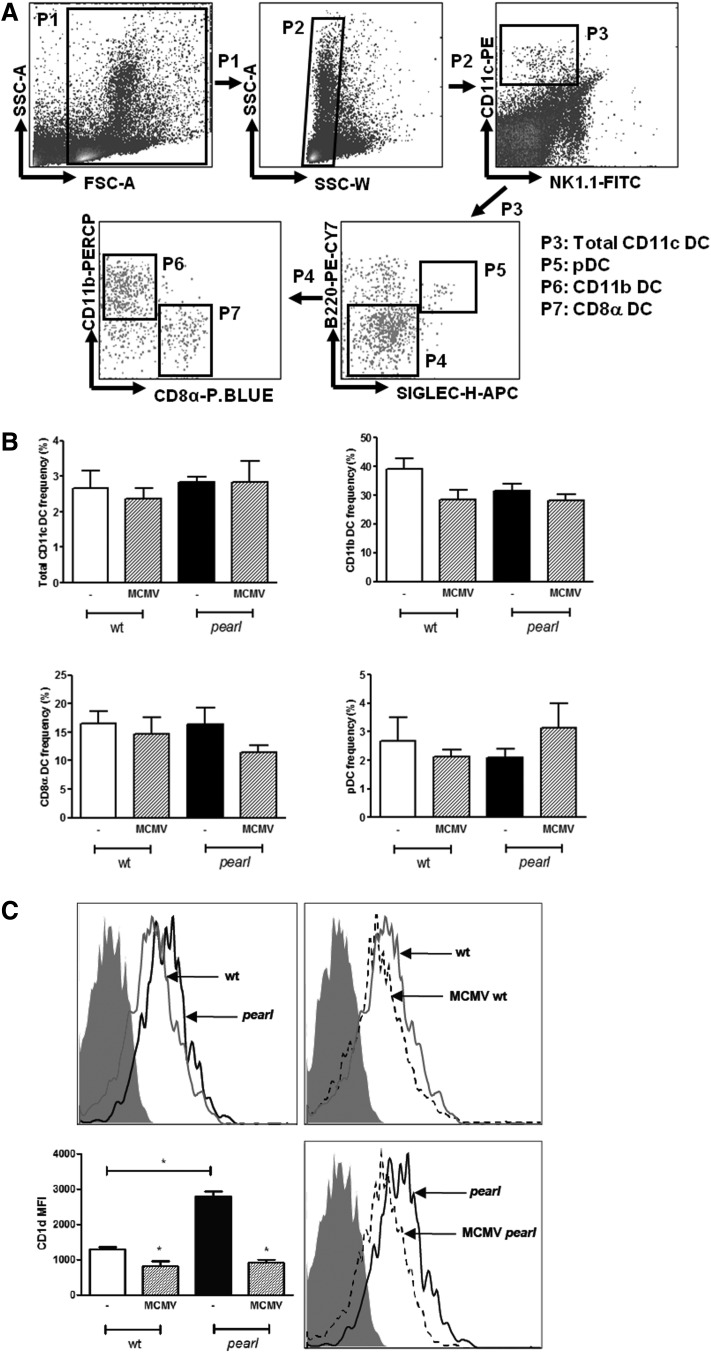
Mouse splenic DC include 3 main populations. The cDC splenic subsets can be identified on the basis of the presence or absence of a CD8α homodimer, while pDC are characterized by high B220 expression (Del Prete and others 2004). Both pDC and cDC play an important role in the antiviral response to MCMV: in addition, there is evidence that changes in their proportion can account for different susceptibilities to viral infections (Andrews and others 2003; Traub and others 2012). To further investigate whether the AP-3 deficiency affects the normal distribution of different splenic DC populations, we characterized the DC subsets for specific cell surface markers (Fig. 2A). DC were derived from wt and pearl mice spleens under steady-state conditions or after infection with MCMV (36 h following challenge with 3×105 plaque-forming unit (PFU) i.p.). As shown in Fig. 2B, the phenotypic characterization of spleen-derived DC from pearl and wt mice revealed similar proportions of CD11c+DC (upper left panel) and comparable frequencies of cDC, both CD11b+CD8α− (upper right panel) and CD11b−CD8α+ (lower left panel), and of B220+Siglec-H+pDC (lower right panel). In addition, pearl mice maintained a stable population of CD11c+ cells following MCMV infection and no significant differences were found in the frequency of CD11b+CD8α−, CD11b−CD8α+, and B220+Siglec-H+ (Fig. 2B).
FIG. 2.
Phenotypic characterization of DC subsets in pearl mice under steady-state conditions or following MCMV infection. Wt and pearl mice were untreated or infected i.p. with 3×105 plaque-forming unit (PFU) of MCMV, and spleens were harvested 36 h later. (A) Splenocytes were analyzed by the FACS CANTO II System (BD Biosciences) for the expression of CD11c, NK 1.1, CD11b, CD8α, B220 (all from BD Biosciences), and Siglec-H (eBioscience). The fluorescence-activated cell sorting gating strategy used is shown. Among the total DC (CD11c+), cDC were identified by the phenotypes CD11b+CD8α− and CD11b−CD8α+, and pDC by the phenotype B220+ Siglec-H+. (B) Frequencies for each population in the spleens of infected and uninfected mice are shown. Data are presented as mean±SEM (bars show averages of 4 mice per group and 1 of 2 independent experiments is shown). (C) Expression of cell surface CD1d by CD11c+ cells from spleens of uninfected and MCMV-infected wt and pearl mice. Histograms are representative plots of 2 independent experiments of 4 mice per group. Isotype control immunoglobulin G is shown as gray filled histograms, untreated wt mice as grey line, and untreated pearl mice as black line, MCMV-infected wt and MCMV-infected pearl mice as dashed line. Mean fluorescence intensity of CD1d on CD11c+ cells from the spleen of uninfected or MCMV-infected wt and pearl mice is shown as mean±SEM (n=4) [(C), low left panel]. Results were analyzed by the 2-tailed Student's t-test. *P<0.05.
The AP-3 complex is also required for normal trafficking of CD1d, a molecule involved in the presentation of lipid antigens to NKT cells and in their selection. CD1d is constitutively expressed on CD11c+ cells and reduced during virus infection (Lin and others 2005). In pearl mice, the abnormal trafficking of CD1d leads to increased expression of CD1d on the surface of different cell types and to a decreased expression in late endosomes coupled with a decreased NKT cell number (Elewaut and others 2003). In agreement with these data, fluorescence-activated cell sorting analysis showed more than a 2-fold increase in CD1d expression levels on the surface of total splenic pearl mice-derived DC compared to wt cells (Fig. 2C upper and lower left panels). However, MCMV infection strongly reduced CD1d expression in pearl mice to levels comparable to those observed in wt mice (Fig. 2C upper and lower right panels).
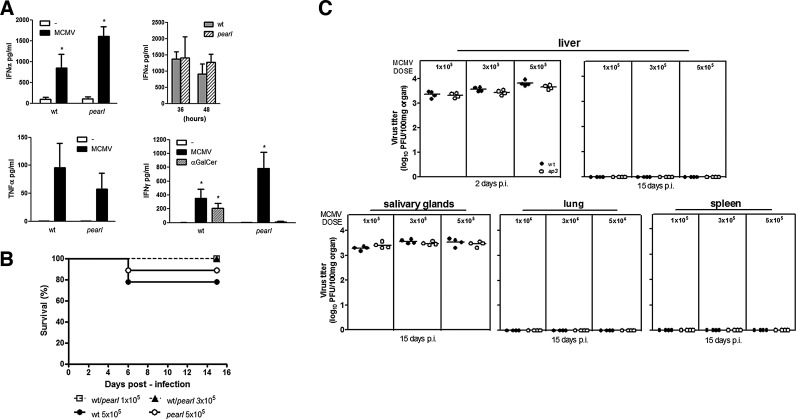
To assess the effects of AP-3 deficiency in the cytokine response to in vivo MCMV infection, levels of IFN-α, TNF-α, and IFN-γ were evaluated by enzyme-linked immunosorbent assay in the sera of wt and pearl mice 36 h after MCMV challenge (3×105 PFU, i.p.), when cytokine production exhibits a sharp peak (Orange and Biron 1996). According to several reports (Dalod and others 2003; Delale and others 2005; Zucchini and others 2008a; Swiecki and others 2010), pDC are essential for the in vivo production of IFN-α during the initial stages of MCMV infection. However, we did not find any difference in the circulating levels of IFN-α in pearl compared to wt mice at 36 h (Fig. 3A upper left panel), despite the defective IFN-α production observed in vitro in pearl mice-derived pDC stimulated with MCMV (Fig. 1A). As shown in Fig. 3A (upper right panel), similar levels of IFN-α were also detected at a later time point (48 h), a time in which IFN-α production is considered non-pDC dependent (Delale and others 2005; Swiecki and others 2010). A mild nonsignificant decrease in TNF-α production was observed in pearl mice compared to wt (Fig. 3A lower left panel). IFN-γ levels were evaluated in the sera of MCMV-infected mice as well as in mice injected with the glycolipid antigen α-galactosylceramide (α-GalCer) (100 ng/mL), which induces NKT cell activation in a CD1d-dependent manner (Kawano and others 1997). Similarly to IFN-α, pearl mice were as efficient as wt animals in IFN-γ production following MCMV infection. In contrast, the IFN-γ levels were strongly reduced in α-GalCer-treated pearl mice compared to wt (Fig. 3A lower right panel), in agreement with the occurrence of a reduced number of Vα14 invariant NKT (Vα14i NKT) cells, which has previously been shown in pearl mice (Elewaut and others 2003).
FIG. 3.
Cytokine production, survival, and viral load in wt and pearl mice during MCMV infection. (A) Serum cytokines in wt and pearl mice during MCMV infection with 3×105 PFU of a salivary gland stock virus i.p. injected. IFN-α (left upper panel) and TNF-α (lower left panel) serum levels were measured by enzyme-linked immunosorbent assay (ELISA) at 36 h. Values are expressed as mean±SEM of 2 independent experiments (4 mice per group). Circulating IFN-α levels of infected mice were also determined at later time point (48 h) (upper right panel) in 2 independent experiments (3 mice per group). Wt and pearl mice were infected with MCMV or injected with α-galactosylceramide (α-GalCer) (100 ng/mL) and analyzed for IFN-γ serum levels at 36 h by ELISA (lower right panel). Data are combined from 2 independent experiments (4 mice per group). (B) Survival curves of wt and pearl mice infected with MCMV. Nine wt or pearl mice were infected i.p. with 1×105, 3×105, or 5×105 PFU and monitored daily for a period of 15 days. The Kaplan–Meier survival analysis model was used to assess the survival of mice in each group. As indicated, the mortality was presented as the percentage of survival mice. (C) Virus production in wt and pearl mice infected with MCMV. The graph represents the virus titers in 4 mice for the group infected i.p. with 1×105, 3×105, or 5×105 PFU of MCMV. The viral loads were evaluated in the liver at 2 and 15 days postinfection (p.i.), and in salivary glands, lungs, and spleens at 15 days p.i. All organs were individually weighed, homogenized in Dulbecco's modified Eagle medium (DMEM)-10% fetal bovine serum as a 10% (w/v) suspension, and centrifuged at 12,000 g×15′ at 4°C. Supernatants were titrated by plaque assay on NIH3T3 cells and the given values were calculated for 100 mg of each organ. Results were analyzed by the 2-tailed Student's t-test. *P<0.05.
To determine the overall susceptibility to MCMV infection, AP-3-deficient mice were challenged with different viral doses. The mortality and viral load in different organs were assessed in comparison with those of wt mice. Upon infection with 1×105 or 3×105 PFU, all wt and AP-3-deficient mice survived (Fig. 3B). At the higher dose, 5×105 PFU, survival of both wt and AP-3-deficient mice was ∼80% (Fig. 3B). The viral titers in multiple tissues of pearl and wt mice infected with different doses of MCMV (1, 3, or 5×105 PFU) were assessed at different postinfection (p.i.) time points. As shown in Fig. 3C, in the liver of pearl and wt mice similar levels of MCMV were titrated at 2 days p.i. Since MCMV replicates in salivary glands during the late stage of infection, the viral titers were measured in salivary glands on day 15 p.i., and no differences were found. Determination of viral titers in the liver, lung, and spleen on day 15 p.i., revealed that pearl mice, as wt animals, were able to reduce MCMV loads, regardless of the dose, below the detection limit.
Taken together, these observations suggest that pearl mice, despite a major defect affecting the in vitro pDC response to TLR7 and TLR9 stimuli, produce normal levels of IFN-α following MCMV infection and control the infection with the same efficiency as wt animals.
The role of pDC in the early innate control varies according to the virus and the infection model (Cervantes-Barragan and others 2012). Several reports explored the role of pDC and IFN-α production in response to MCMV infection, by using different models of pDC depletion or functional impairment in the absence of the TLR9/MyD88 pathway. For example, pDC depletion in IkarosL/L mice (Allmann and others 2006) or BDCA2-DTR transgenic mice (Swiecki and others 2010) resulted in deficient production of IFN-α at early time points after MCMV infection. However, IFN-α production was restored at later time points (Allmann and others 2006; Swiecki and others 2010) by cells other than pDC. The effects of pDC depletion on the overall anti-MCMV response seem to be dependent on viral doses. In fact, at high viral loads, BDCA2-DTR mice, similarly to what we observed in pearl mice, were efficient in containing MCMV replication (Swiecki and others 2010). A less efficient control of viral replication in the IkarosL/L pDC depletion model may be due to the potential impairment of other hematopoietic lineages (Allmann and others 2006).
The role of TLR9 in MCMV infection, in the presence or in the absence of pDC, has been carefully characterized by Delale and others (2005). Interestingly, these authors observed that TLR9−/− mice maintained a pDC-dependent IFN-α production, in response to MCMV infection, notwithstanding the in vitro defective IFN-α secretion. These findings, in line with our data, suggest that some compensatory mechanisms may occur during the in vivo response to MCMV infection. It is therefore conceivable that in pearl mice, other hematopoietic and nonhematopoietic cells (ie, lymphoid-tissue stromal cells) could contribute to the overall in vivo production of IFN-α (Andoniou and others 2005; Delale and others 2005). In this regard, it has been shown that cDC are able to produce type I IFN in a TLR9-independent manner, and that TLR2 and TLR3 may play a role in the defense against MCMV infection (Tabeta and others 2004; Barbalat and others 2009). Moreover, a TLR-independent production of IFN-α by cDC in response to MCMV has been reported to occur through the retinoic acid-inducible gene 1 (Yoneyama and others 2004). Interestingly, although TLR7 mediates recognition of ssRNA virus and TLR9 sense MCMV, Zucchini and others (2008b) reported that TLR7 and TLR9 redundantly affect IFN-α production by pDC during MCMV infection in vivo. These authors observed a decrease in the IFN-α production in TLR7/9 double-deficient mice that, however, was not detected in TLR9−/− mice (Zucchini and others 2008b). This is in agreement with our and Delale's data, and further underlines the occurrence of TLR9-independent mechanisms. However, unlike TLR9−/− mice and TLR7/9 double-deficient mice, which were more susceptible to MCMV infection especially at high challenging doses (Delale and others 2005; Zucchini and others 2008b), we observed that pearl mice did not show an increased susceptibility to MCMV challenge when compared to wt controls. This difference may stem from the different players that had been knocked out in the various experimental mouse models (ie, AP-3 vs. TLR9). The AP-3 complex interacts with cleaved TLR9 and facilitates its trafficking to LAMP2 lysosome-related organelles (LRO), whereby TLR9 can engage TRAF3 and IRF7 and induce transcription of IFN genes. However, in AP-3-deficient cells, TLR9 is unable to traffic to a specialized LAMP2+ LRO and to engage IRF7, but enters nuclear factor-kappa B endosome becoming competent to activate certain proinflammatory cytokine genes (Sasai and others 2010). Thus, it is conceivable that the ability of TLR9 to sense MCMV DNA and to stimulate the expression of inflammatory cytokines, even in AP-3-deficient mice, can account for the initiation of an antiviral response that leads to the control of MCMV infection. Furthermore, the observation of an efficient anti-MCMV response in AP-3-deficient mice is in agreement with data from Jessen and others (2013), who reported that pearl mice infected with lymphocytic choriomeningitis virus control infection and clear the virus from all organs.
In conclusion, our results with the AP-3-deficient mouse model, while confirming previous results obtained with different experimental models on the limited role of pDC in contributing to the overall response to MCMV infection, support the view that the impaired immune functions caused by mutation in the ADTB3A gene have a minimal impact on the generation of protective antiviral responses.
Acknowledgments
This work was financially supported by the National Research Projects (PRIN 2010–2011, grant no. 2010PHT9NF and Ex-60%) from MIUR (Ministero Istruzione, Università e Ricerca) and by the FP7 project HLH-Cure.
Author Disclosure Statement
No competing financial interests exist.
References
- Ahmed M, Mitchell LM, Puckett S, Brzoza-Lewis KL, Lyles DS, Hiltbold EM. 2009. Vesicular stomatitis virus M protein mutant stimulates maturation of Toll-like receptor 7 (TLR7)-positive dendritic cells through TLR-dependent and -independent mechanisms. J Virol 83(7):2962–2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman D, Dalod M, Asselin-Paturel C, Delale T, Robbins SH, Trinchieri G, Biron CA, Kastner P, Chan S. 2006. Ikaros is required for plasmacytoid dendritic cell differentiation. Blood 108(13):4025–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, Delale T, Stacey KJ, Trinchieri G, Degli-Esposti MA. 2005. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol 6(10):1011–1019 [DOI] [PubMed] [Google Scholar]
- Andrews DM, Andoniou CE, Granucci F, Ricciardi-Castagnoli P, Degli-Esposti MA. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat Immunol 2(11):1077–1084 [DOI] [PubMed] [Google Scholar]
- Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. 2003. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol 4(2):175–181 [DOI] [PubMed] [Google Scholar]
- Badolato R, Parolini S. 2007. Novel insights from adaptor protein 3 complex deficiency. J Allergy Clin Immunol 120(4):735–741 [DOI] [PubMed] [Google Scholar]
- Barbalat R, Lau L, Locksley RM, Barton GM. 2009. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 10(11):1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, Ross C, Li X, Smart NG, Beutler B. 2010. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 107(46):19973–19978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, Ludewig B, Reizis B. 2012. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci U S A 109(8):3012–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, Biron CA. 2003. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J Exp Med 197(7):885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EE, Kastner P, Chan S, Akira S, Vicari A, Biron CA, Trinchieri G, Brière F. 2005. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J Immunol 175(10):6723–6732 [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC. 2009. AP-3-dependent trafficking and disease: the first decade. Curr Opin Cell Biol 21(4):552–559 [DOI] [PubMed] [Google Scholar]
- Del Prete A, Shao WH, Mitola S, Santoro G, Sozzani S, Haribabu B. 2007. Regulation of dendritic cell migration and adaptive immune response by leukotriene B4 receptors: a role for LTB4 in up-regulation of CCR7 expression and function. Blood 109(2):626–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete A, Vermi W, Dander E, Otero K, Barberis L, Luini W, Bernasconi S, Sironi M, Santoro A, Garlanda C, Facchetti F, Wymann MP, Vecchi A, Hirsch E, Mantovani A, Sozzani S. 2004. Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. EMBO J 23(17):3505–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewaut D, Lawton AP, Nagarajan NA, Maverakis E, Khurana A, Honing S, Benedict CA, Sercarz E, Bakke O, Kronenberg M, Prigozy TI. 2003. The adaptor protein AP-3 is required for CD1d-mediated antigen presentation of glycosphingolipids and development of Valpha14i NKT cells. J Exp Med 198(8):1133–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Seymour AB, Jiang S, To A, Peden AA, Novak EK, Zhen L, Rusiniak ME, Eicher EM, Robinson MS, Gorin MB, Swank RT. 1999. The β3A subunit gene (Ap3b1) of the AP-3 adaptor complex is altered in the mouse hypopigmentation mutant pearl, a model for Hermansky-Pudlak syndrome and night blindness. Hum Mol Genet 8(2):323–330 [DOI] [PubMed] [Google Scholar]
- Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. 2004. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci U S A 101(31):11416–11421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen B, Bode SF, Ammann S, Chakravorty S, Davies G, Diestelhorst J, Frei-Jones M, Gahl WA, Gochuico BR, Griese M, Griffiths G, Janka G, Klein C, Kögl T, Kurnik K, Lehmberg K, Maul-Pavicic A, Mumford AD, Pace D, Parvaneh N, Rezaei N, de Saint Basile G, Schmitt-Graeff A, Schwarz K, Karasu GT, Zieger B, Zur Stadt U, Aichele P, Ehl S. 2013. The risk of hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type 2. Blood 121(15):2943–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278(5343):1626–1629 [DOI] [PubMed] [Google Scholar]
- Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S. 2003. Pathogenesis of murine cytomegalovirus infection. Microbes Infect 5(13):1263–1277 [DOI] [PubMed] [Google Scholar]
- Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21(1):107–119 [DOI] [PubMed] [Google Scholar]
- Lin Y, Roberts TJ, Spence PM, Brutkiewicz RR. 2005. Reduction in CD1d expression on dendritic cells and macrophages by an acute virus infection. J Leukoc Biol 77(2):151–158 [DOI] [PubMed] [Google Scholar]
- Mantegazza AR, Guttentag SH, El-Benna J, Sasai M, Iwasaki A, Shen H, Laufer TM, Marks MS. 2012. Adaptor protein-3 in dendritic cells facilitates phagosomal toll-like receptor signaling and antigen presentation to CD4(+) T cells. Immunity 36(5):782–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Biron CA. 1996. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol 156(12):4746–4756 [PubMed] [Google Scholar]
- Rahman S, Khan ZK, Jain P. 2011. The tug-of-war between dendritic cells and human chronic viruses. Int Rev Immunol 30(5–6):341–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera L, Gariglio M, Valente G, Müllbacher A, Museteanu C, Landolfo S, Simon MM. 2000. Murine cytomegalovirus replication in salivary glands is controlled by both perforin and granzymes during acute infection. Eur J Immunol 30(5):1350–1355 [DOI] [PubMed] [Google Scholar]
- Rölle A, Olweus J. 2009. Dendritic cells in cytomegalovirus infection: viral evasion and host countermeasures. APMIS 117(5–6):413–426 [DOI] [PubMed] [Google Scholar]
- Sasai M, Linehan MM, Iwasaki A. 2010. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science 329(5998):1530–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani S, Vermi W, Del Prete A, Facchetti F. 2010. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol 31(7):270–277 [DOI] [PubMed] [Google Scholar]
- Steinberg C, Eisenächer K, Gross O, Reindl W, Schmitz F, Ruland J, Krug A. 2009. The IFN regulatory factor 7-dependent type I IFN response is not essential for early resistance against murine cytomegalovirus infection. Eur J Immunol 39(4):1007–1018 [DOI] [PubMed] [Google Scholar]
- Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. 2010. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity 33(6):955–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A 101(10):3516–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub S, Demaria O, Chasson L, Serra F, Desnues B, Alexopoulou L. 2012. Sex bias in susceptibility to MCMV infection: implication of TLR9. PLoS One 7(9):e45171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5(7):730–737 [DOI] [PubMed] [Google Scholar]
- Zucchini N, Bessou G, Robbins SH, Chasson L, Raper A, Crocker PR, Dalod M. 2008a. Individual plasmacytoid dendritic cells are major contributors to the production of multiple innate cytokines in an organ-specific manner during viral infection. Int Immunol 20(1):45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchini N, Bessou G, Traub S, Robbins SH, Uematsu S, Akira S, Alexopoulou L, Dalod M. 2008b. Cutting edge: overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. J Immunol 180(9):5799–5803 [DOI] [PubMed] [Google Scholar]