Figure 3.
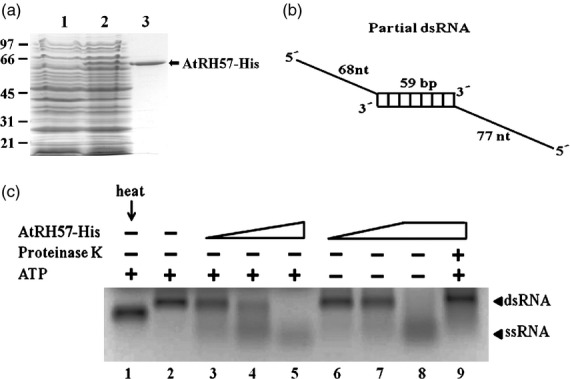
Over-expression and activity assay of AtRH57-His protein. (a) Over-expression and purification of AtRH57-His protein in Escherichia coli. The expressed vector only (lane 1) and AtRH57-His fusion protein (lane 2) were extracted, affinity-purified (lane 3) using Ni2+-NTA-agarose, fractionated, and then stained with Coomassie blue. Marker protein sizes are indicated at the left. (b) Schematic representation of the partial dsRNA substrate. The RNA substrate contains a 59-bp duplex region, with 68 and 77 nucleotide long single-stranded regions at the 5′ ends. (c) In vitroRNA helicase activity assay of AtRH57-His protein. Various amounts of AtRH57-His protein: 40 ng (lanes 3 and 6), 90 ng (lanes 4 and 7), and 376 ng (lanes 5, 8, and 9) were incubated with 1.0 μg partial dsRNA in 20 μl of helicase buffer at 30°C for 1 h and separated by 1.2% formaldehyde gel electrophoresis. Reactions were either added with (lanes 1–5 and 9) or without (lanes 6–8) 1 mmATP. The reaction mixture in lane 9 was pre-treated with proteinase K prior to incubation with partial dsRNA. Partial dsRNA was treated either with (lane 1) or without (lane 2) heat as controls. dsRNA and ssRNA bands were subsequently visualized by ethidium bromide (EtBr) staining.
