Figure 7.
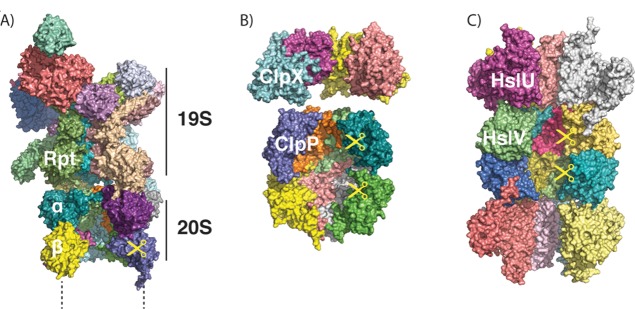
ATP-dependent proteases share a common architecture. (A) Structure of the proteasome, as modeled from cryo-electron microscopy (PDB ID 4C0V; ATPγS bound). Two α, two β, and two Rpt subunits were removed to allow visualization of the interior. Only one-half (one α, one β ring) of the 20S core protease particle and one 19S regulatory particle are shown. (B) Structures of ClpX (PDB ID 3HWS; nucleotide-free) and ClpP (PDB ID 1Y6G), showing the interior of the barrel. Four out of six subunits of ClpX and four out of seven subunits of ClpP per ring are shown. (C) Structure of HslUV (PDB ID 1G3I; ATP-bound), showing the interior of the barrel. Four out of six subunits of HslU and V per ring are shown.
