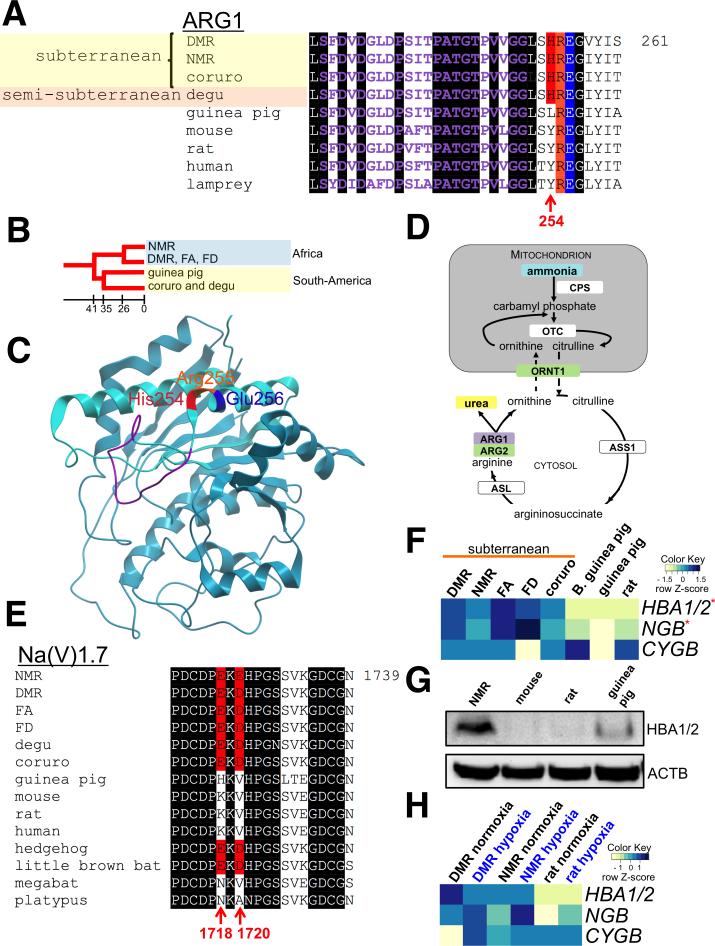
Figure 2. Subterranean adaptations in hystricognath rodents.
(A) Subterranean rodents share a charged residue at position 254 of arginase 1 (ARG1). The manganese-binding site, residues critical for enzyme trimer assembly (Arg255 and Glu256), and unique His254 changes are highlighted in purple, orange, blue and red, respectively. Identical residues in vertebrates are shaded in black. (B) Phylogenetic relationship of hystricognath rodent lineages examined in this study. Approximate divergence times (Myr) are indicated. (C) Structural model of human ARG1 monomer. Residues are highlighted as in (A). (D) Schematic representation of the roles of components of the urea cycle with altered sequence (purple box) or expression in NMR and DRM (green boxes). CPS1, carbamoyl phosphate synthase 1; Ornithine transcarbamylase, OTC; ORNT1, ornithine transporter 1; ASS1, argininosuccinate synthase; ASL, argininosuccinate lyase; ARG1, arginase 1; ARG2, arginase 2. (E) Species of hypercapnic habitats share a negatively charged three-residue motif in the Na(V)1.7 sodium channel protein. Acidic amino acid residues in the motif, corresponding to amino acids 1718 and 1720 of the human sequence, are shown in red. Identical residues in vertebrates are shaded in black. (F). Heatmap of globin expression in normoxic rodent brains. Scaled log2 transformed normalized read counts, denoted as the row Z-score, is plotted in beige–blue color with blue indicating high expression and beige indicating low expression, respectively. B. guinea pig, Brazilian guinea pig; HBA1/2, hemoglobin α; NGB, neuroglobin; CYGB, cytoglobin. Red stars indicate differentially expressed genes in subterranean rodents. (G) Western blot of hemoglobin α in normoxic rodent brains with antibodies against the mouse protein. (H) Comparison of globin gene expression under normoxia (21% O2) and hypoxia (8% O2 over 8 hours). Annotated as in (F). See also Figure S2.