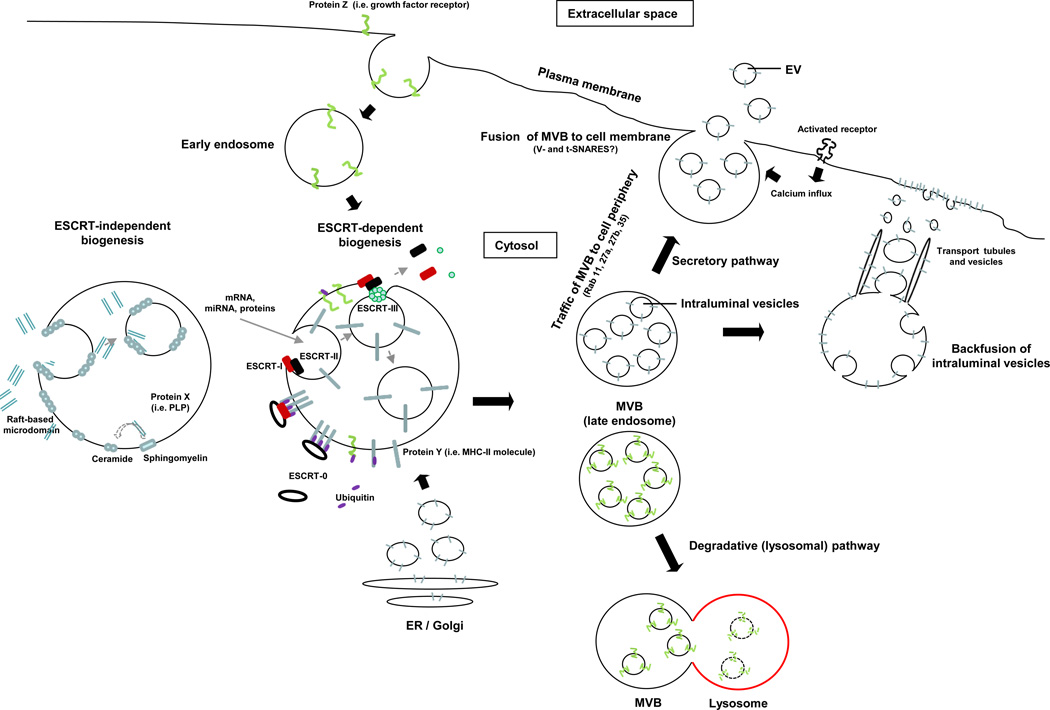
Figure 1. Biogenesis of extracellular vesicles.
EVs are generated as ILVs in MVBs by ESCRT-dependent or –independent mechanisms. Proteins transported from the Golgi (i.e. MHC Class-II molecules), or internalized from the cell surface (i.e. activated growth factor receptors) are ubiquitylated on their cytosolic domains. However, not all proteins such as MHC Class-II required ubiquitinylation for targeting to vesicles. The ESCRT-0 complex recognizes the ubiquitylated proteins on the cytosolic side of the endosome / MVB membrane, segregates the proteins into microdomains, and binds the ESCRT-I complex, which in turn recruits ESCRT–II subunits. ESCRT-I and –II initiate the reverse budding of the nascent ILVs within MVBs. At this time, cytosolic RNAs and proteins have direct access into the interior of the forming vesicles. Next, the ESCRT-II complex recruits ESCRT-III subunits inside the neck of the nascent ILVs, which results in their cleavage into free vesicles. The free ubiquitin molecules and ESCRT subunits are released into the cytosol for recycling. Certain proteins (i.e. the proteolipid protein, PLP) are sorted into ILVs independently of the ESCRT machinery through raft-based microdomains rich in sphingolipids, from which ceramides are formed by sphingomyelinases. Ceramide induces coalescence of the microdomains and triggers ILV formation. The dashed line indicates that the role of ceramide in ILV formation is still controversial. The MVBs then follow either the secretory or degradative pathway. In the former, MVBs traffic to the cell periphery and fuse with the cell membrane, releasing the ILVs (now termed EV) constitutively, or following activation of surface receptors that trigger calcium influx. In the degradative route, MVBs released the ILVs into lysosomes. The lysosomal pathway is critical for limiting signaling of activated growth factor receptors. It is likely that differences in the MVBs confer the route of traffic.