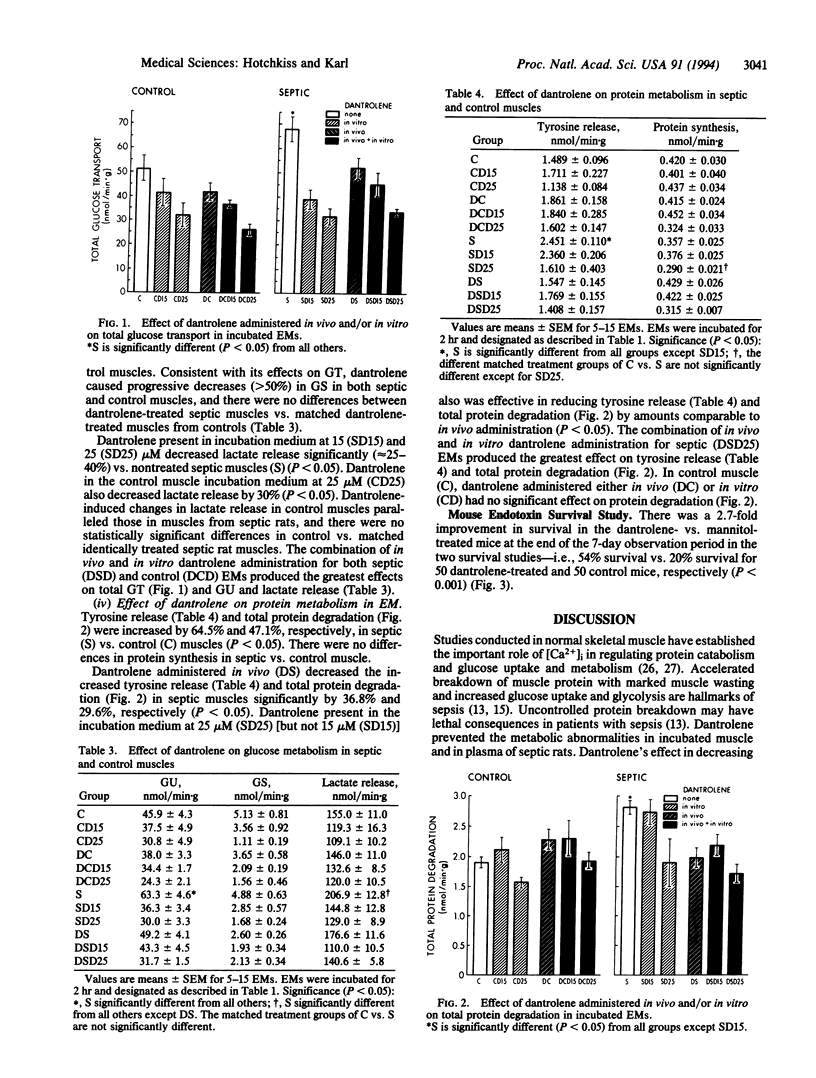
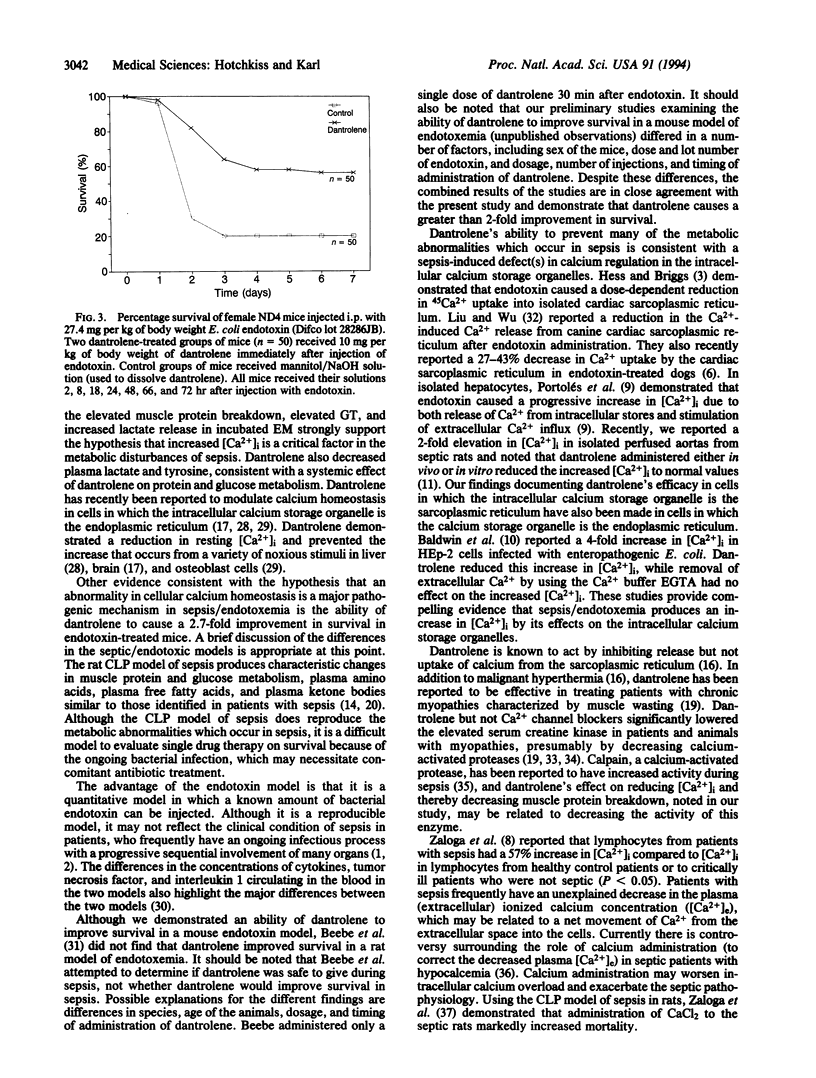
Abstract
Sepsis is the systemic inflammatory response resulting from serious infection and is the most common cause of death in intensive care units. Intracellular free calcium concentration ([Ca2+]i) is an important regulator of numerous cellular processes and when increased excessively may act as a potent cellular toxin. To determine if [Ca2+]i is responsible for the major metabolic changes which are hallmarks of sepsis, we examined if sodium dantrolene, a drug which decreases release of calcium from sarcoplasmic reticulum, affected the metabolic abnormalities in plasma and epitrochlearis muscles of rats made septic by cecal ligation and perforation. Dantrolene when added in vitro or when given in vivo decreases many of the metabolic hallmarks of sepsis--i.e., muscle protein breakdown approximately 30%, muscle glucose transport approximately 38%, muscle lactate formation approximately 28%, and plasma lactate approximately 29% (P < 0.05). In addition, we examined the ability of dantrolene to improve survival in a mouse model of endotoxemia. Dantrolene caused > 2-fold improvement in survival when it was administered concurrently with endotoxin (54% vs. 20% survival in dantrolene-treated and control mice, respectively (P < 0.001). Our results are consistent with the hypothesis that an increase in [Ca2+]i plays an important role in the metabolic abnormalities which occur during sepsis and that dantrolene administration may be an effective therapeutic strategy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. J., Ward W., Aitken A., Knutton S., Williams P. H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991 May;59(5):1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow C. E., Schmidt M. F., Hassinger T. D., Schwab M. E., Kater S. B. Role of intracellular calcium in NI-35-evoked collapse of neuronal growth cones. Science. 1993 Jan 1;259(5091):80–83. doi: 10.1126/science.8418499. [DOI] [PubMed] [Google Scholar]
- Baracos V., Greenberg R. E., Goldberg A. L. Influence of calcium and other divalent cations on protein turnover in rat skeletal muscle. Am J Physiol. 1986 Jun;250(6 Pt 1):E702–E710. doi: 10.1152/ajpendo.1986.250.6.E702. [DOI] [PubMed] [Google Scholar]
- Beebe D. S., Belani K. G., Tuohy S. E., Sweeney M. F., Gillingham K., Komanduri V., Palahniuk R. J. Is dantrolene safe to administer in sepsis? The effect of dantrolene after endotoxin administration in dogs and rats. Anesth Analg. 1991 Sep;73(3):289–294. doi: 10.1213/00000539-199109000-00011. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya J., Thompson K., Sayeed M. M. Calcium-dependent and calcium-independent protease activities in skeletal muscle during sepsis. Circ Shock. 1991 Oct;35(2):117–122. [PubMed] [Google Scholar]
- Cerra F. B. Hypermetabolism, organ failure, and metabolic support. Surgery. 1987 Jan;101(1):1–14. [PubMed] [Google Scholar]
- Chernow B. Calcium: does it have a therapeutic role in sepsis? Crit Care Med. 1990 Aug;18(8):895–896. [PubMed] [Google Scholar]
- Clausen T., Elbrink J., Dahl-Hansen A. B. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. IX. The role of cellular calcium in the activation of the glucose transport system in rat soleus muscle. Biochim Biophys Acta. 1975 Jan 28;375(2):292–308. doi: 10.1016/0005-2736(75)90197-2. [DOI] [PubMed] [Google Scholar]
- Clowes G. H., Jr, George B. C., Villee C. A., Jr, Saravis C. A. Muscle proteolysis induced by a circulating peptide in patients with sepsis or trauma. N Engl J Med. 1983 Mar 10;308(10):545–552. doi: 10.1056/NEJM198303103081001. [DOI] [PubMed] [Google Scholar]
- Davis T. A., Karl I. E. Response of muscle protein turnover to insulin after acute exercise and training. Biochem J. 1986 Dec 15;240(3):651–657. doi: 10.1042/bj2400651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. F., Snyder Y. M., Butler L. D., Zuckerman S. H. Differential expression of interleukin-1 and tumor necrosis factor in murine septic shock models. Circ Shock. 1989 Dec;29(4):279–290. [PubMed] [Google Scholar]
- Frandsen A., Schousboe A. Mobilization of dantrolene-sensitive intracellular calcium pools is involved in the cytotoxicity induced by quisqualate and N-methyl-D-aspartate but not by 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionate and kainate in cultured cerebral cortical neurons. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2590–2594. doi: 10.1073/pnas.89.7.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. II. The precursor role of amino acids in alanine and glutamine synthesis. J Biol Chem. 1976 Feb 10;251(3):836–843. [PubMed] [Google Scholar]
- Harrison G. G. Malignant hyperthermia. Dantrolene--dynamics and kinetics. Br J Anaesth. 1988 Feb;60(3):279–286. doi: 10.1093/bja/60.3.279. [DOI] [PubMed] [Google Scholar]
- Haymond M. W., Karl I. E., Clarke W. L., Pagliara A. S., Santiago J. V. Differences in circulating gluconeogenic substrates during short-term fasting in men, women, and children. Metabolism. 1982 Jan;31(1):33–42. [PubMed] [Google Scholar]
- Hess M. L., Briggs F. N. The effect of Gram negative endotoxin on the calcium uptake activity of sarcoplasmic reticulum isolated from canine myocardium. Biochem Biophys Res Commun. 1971 Nov;45(4):917–923. doi: 10.1016/0006-291x(71)90425-6. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. S., Karl I. E. Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis. JAMA. 1992 Mar 18;267(11):1503–1510. [PubMed] [Google Scholar]
- Karl I. E., Gavin J. R., 3rd, Levy J. Effect of insulin on glucose utilization in epitrochlearis muscle of rats with streptozocin-induced NIDDM. Diabetes. 1990 Sep;39(9):1106–1115. doi: 10.2337/diab.39.9.1106. [DOI] [PubMed] [Google Scholar]
- Liu M. S., Wu L. L. Reduction in the Ca2(+)-induced Ca2+ release from canine cardiac sarcoplasmic reticulum following endotoxin administration. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1248–1254. doi: 10.1016/0006-291x(91)91555-q. [DOI] [PubMed] [Google Scholar]
- Meldrum D. R., Ayala A., Perrin M. M., Ertel W., Chaudry I. H. Diltiazem restores IL-2, IL-3, IL-6, and IFN-gamma synthesis and decreases host susceptibility to sepsis following hemorrhage. J Surg Res. 1991 Aug;51(2):158–164. doi: 10.1016/0022-4804(91)90088-4. [DOI] [PubMed] [Google Scholar]
- Mine T., Kojima I., Kimura S., Ogata E. Assessment of the role of Ca2+ mobilization from intracellular pool(s), using dantrolene, in the glycogenolytic action of alpha-adrenergic stimulation in perfused rat liver. Biochim Biophys Acta. 1987 Feb 18;927(2):229–234. doi: 10.1016/0167-4889(87)90139-x. [DOI] [PubMed] [Google Scholar]
- Mészáros K., Lang C. H., Bagby G. J., Spitzer J. J. Contribution of different organs to increased glucose consumption after endotoxin administration. J Biol Chem. 1987 Aug 15;262(23):10965–10970. [PubMed] [Google Scholar]
- Nicotera P., Bellomo G., Orrenius S. Calcium-mediated mechanisms in chemically induced cell death. Annu Rev Pharmacol Toxicol. 1992;32:449–470. doi: 10.1146/annurev.pa.32.040192.002313. [DOI] [PubMed] [Google Scholar]
- Parrillo J. E. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993 May 20;328(20):1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- Portolés M. T., Ainaga M. J., Municio A. M., Pagani R. Intracellular calcium and pH alterations induced by Escherichia coli endotoxin in rat hepatocytes. Biochim Biophys Acta. 1991 Mar 19;1092(1):1–6. doi: 10.1016/0167-4889(91)90170-3. [DOI] [PubMed] [Google Scholar]
- Quinlan J. G., Johnson S. R., Samaha F. J. Dantrolene normalizes serum creatine kinase in MDX mice. Muscle Nerve. 1990 Mar;13(3):268–269. doi: 10.1002/mus.880130316. [DOI] [PubMed] [Google Scholar]
- Reid I. R., Civitelli R., Halstead L. R., Avioli L. V., Hruska K. A. Parathyroid hormone acutely elevates intracellular calcium in osteoblastlike cells. Am J Physiol. 1987 Jul;253(1 Pt 1):E45–E51. doi: 10.1152/ajpendo.1987.253.1.E45. [DOI] [PubMed] [Google Scholar]
- Rose S., Thompson K. D., Sayeed M. M. Ca(2+)-related hepatocellular alterations during intra-abdominal sepsis. Am J Physiol. 1992 Sep;263(3 Pt 2):R553–R558. doi: 10.1152/ajpregu.1992.263.3.R553. [DOI] [PubMed] [Google Scholar]
- Sayeed M. M., Maitra S. R. Effect of diltiazem on altered cellular calcium regulation during endotoxic shock. Am J Physiol. 1987 Oct;253(4 Pt 2):R549–R554. doi: 10.1152/ajpregu.1987.253.4.R549. [DOI] [PubMed] [Google Scholar]
- Song S. K., Karl I. E., Ackerman J. J., Hotchkiss R. S. Increased intracellular Ca2+: a critical link in the pathophysiology of sepsis? Proc Natl Acad Sci U S A. 1993 May 1;90(9):3933–3937. doi: 10.1073/pnas.90.9.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. R., Westwood T., Regen C. M., Steinhardt R. A. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988 Oct 20;335(6192):735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi I., Hatake K., Kakishita E., Nagai K. Diminution of contractile response of the aorta from endotoxin-injected rats. Eur J Pharmacol. 1987 Sep 2;141(1):117–122. doi: 10.1016/0014-2999(87)90417-1. [DOI] [PubMed] [Google Scholar]
- Wichterman K. A., Baue A. E., Chaudry I. H. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980 Aug;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Wu L. L., Liu M. S. Impaired calcium uptake by cardiac sarcoplasmic reticulum and its underlying mechanism in endotoxin shock. Mol Cell Biochem. 1991 Nov 13;108(1):9–17. doi: 10.1007/BF00239537. [DOI] [PubMed] [Google Scholar]
- Zaloga G. P., Sager A., Black K. W., Prielipp R. Low dose calcium administration increases mortality during septic peritonitis in rats. Circ Shock. 1992 Jul;37(3):226–229. [PubMed] [Google Scholar]
- Zaloga G. P., Washburn D., Black K. W., Prielipp R. Human sepsis increases lymphocyte intracellular calcium. Crit Care Med. 1993 Feb;21(2):196–202. doi: 10.1097/00003246-199302000-00009. [DOI] [PubMed] [Google Scholar]


