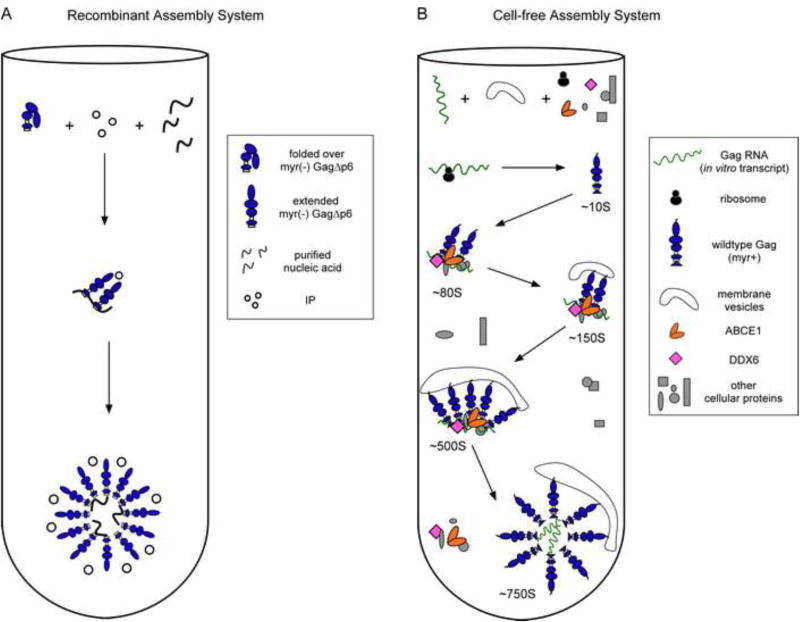
Fig. 4. Comparison of the pathways for assembly in the recombinant and cell-free assembly systems.
(A) The recombinant assembly system contains purified nucleic acids, an inositol phosphate-related compound (IP), and a purified, fully folded, recombinant myr(-)GagΔp6. In solution, myr(-)GagΔp6 is thought to exist in a folded-over form. Addition of IP and oligomerization of Gag converts Gag to an extended form resulting in formation of spherical structures that closely resemble HIV-1 immature capsids. The only intermediate in the pathway is likely a dimer. (B) In the cell-free system, as in cells, Gag progresses through an energy-dependent, host-catalyzed pathway of assembly intermediates (∼10S, ∼80S, ∼150S, and ∼500S complexes), culminating in formation of the ∼750S completed immature capsid. RNAs are found in ribonucleoprotein complexes (RNPs). A cellular extract and purified exogenous components provide factors needed for Gag translation and assembly. Gag mRNA is translated by ribosomes from the extract to produce WT myristoylated Gag de novo. Newly synthesized Gag co-opts host proteins to form the assembly intermediates. Based on studies in the cell-free system and/or cells, the assembly intermediates contain the cellular facilitators of assembly ABCE1 and DDX6. Intact membranes, likely present as vesicles, and myristoylation of Gag are required for progression past the ∼80S intermediate. References are in the text.