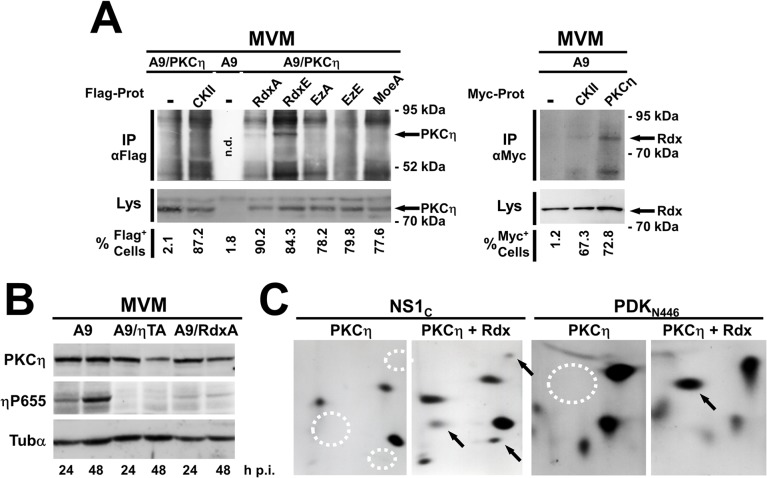
Fig 2. Rdx interacts with PKCη and controls its activity and substrate specificity.
(A, B) A9 cells and derivatives expressing the gene encoding the indicated variant protein under the control of the NS1-inducible P38 promoter were infected with MVM (30 pfu/cell) and analyzed at the indicated times p.i. (A) Rdx interacts physically with PKCη inside cells. Left panel: Cell lines expressing MycPKCη (PKCη) alone or together with Flag-tagged CKIIαE81A (CKII), RdxT564A(Rdxa), RdxT564E (RdxE), EzT566A (EzA), EzT566E (EzE), or MoeT547A (MoeA), were harvested 36 h p.i. Co-immunoprecipitation assays were performed under non-denaturing conditions with mouse monoclonal Flag-tag-specific M2 antibodies. Immunoprecipitates (IPαFlag) and, for comparison, whole-cell lysates (Lys) were analyzed by western blotting with rabbit anti-Myc antibodies to detect MycPKCη. The percentage of Flag-positive cells in these lines was determined by immunofluorescence with M2 antibodies (% Flag+ cells). Arrows indicate the position of MycPKCη in CoIPs. n.d. stands for “not determined”. Right panel: A9, and cell lines expressing MycPKCη or MycCKIIα were harvested 36 h p.i. Co-immunoprecipitation assays were performed under non-denaturing conditions with anti-Myc antibodies. Immunoprecipitates (IPαMyc) and, for comparison, whole-cell lysates (Lys) were analyzed by western blotting with goat anti-Rdx antibodies to detect endogenous radixin. The percentage of Myc-positive cells in these lines was determined by immunofluorescence with anti-Myc antibodies (% Myc+ cells). Arrows indicate the position of Rdx in CoIPs (B) Rdx controls the activity of PKCη in MVM-infected A9 cells. A9 cells and derivatives expressing dominant-negative PKCηT512A (ηTA) or RdxT564A (RdxA) were harvested at the indicated times p.i. and analyzed by western blotting. As a measure of endogenous PKCη activity, the amount of PKCη auto-phosphorylated at T655 (ηP655) was estimated as compared to the total amount of the kinase (PKCη). The loading control was α-tubulin (Tubα). (C) Radixin controls the substrate specificity of PKCη. The MVM NS1 trans-activation domain, aa 545–672 (NS1C) and C-terminally truncated PDK-1N446 were phosphorylated in vitro by PKCη alone (PKCη) or with radixin (PKCη/Rdx) and their tryptic phosphopeptides were detected. Peptides labeled specifically in the presence of Rdx are indicated with arrows (presence) or dotted circles (absence).