Figure 6. Models of Chaperoned Ubiquitination Complexes in ADP- and ATP-Bound States.
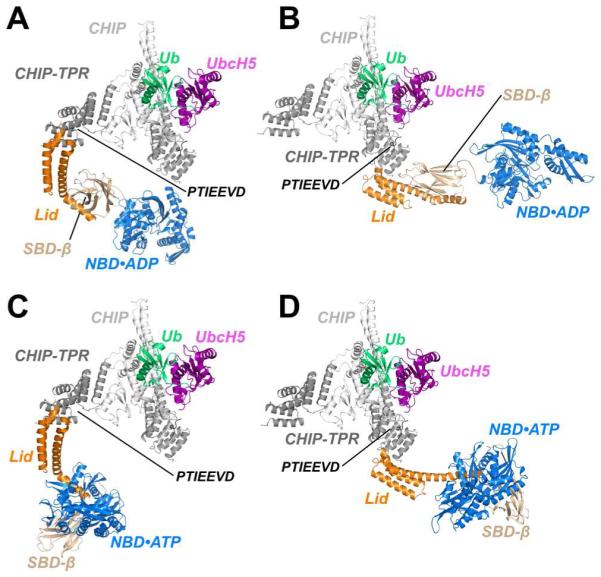
(A) A model of Hsp70, based on the structure of ADP-bound E. coli DnaK (Bertelsen et al., 2009) is fully compatible with binding to the TPR domain of CHIP protomer with an occluded U-box. CHIP, CHIP-TPR, UbcH5, Ub, Hsc70-Lid, Hsc70-Tail, Hsc70-SBDβ and Hsc70-NBD are colored white, grey, purple, green, orange, black, wheat and blue, respectively.
(B) ADP-bound Hsp70 is also compatible with binding to TPR domain of CHIP protomer with accessible U-box.
(C) A model of ATP-bound Hsp70, based on the structure of DnaK in the ATP-bound form (Qi et al., 2013), is compatible with binding to CHIP via the TPR domain of the protomer with an occluded U-box.
(D) The CHIP-TPR from a protomer with an accessible U-box is also compatible with binding to ATP-bound Hsp70.
