Fig 2. Git1 phosphorylation at Tyr-554 weakened its association with Hic-5.
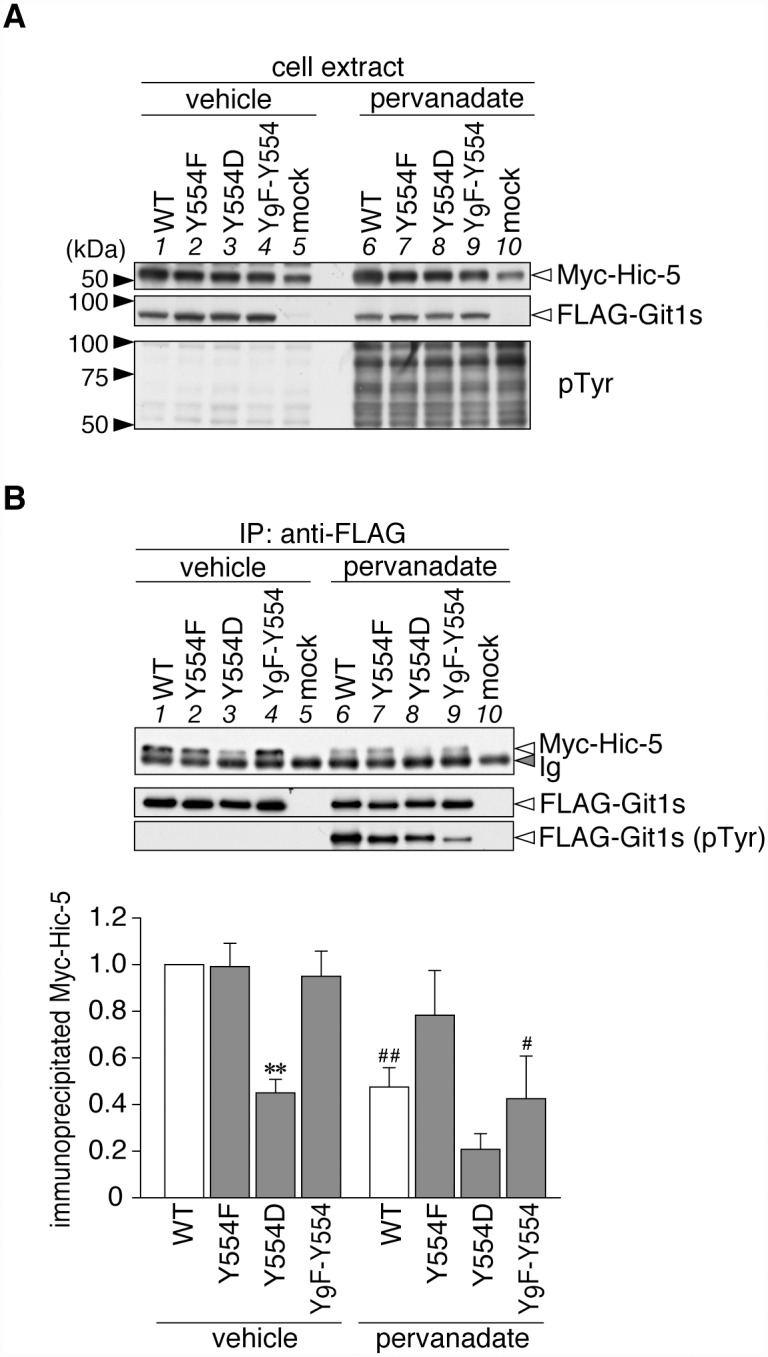
A, Western blotting of protein expression levels, and tyrosine phosphorylation of all proteins in HEK293T cells expressing FLAG-tagged Git1 proteins (Fig. 1A) together with Myc-tagged Hic-5. Cells were treated with 100 μM pervanadate or vehicle for 15 min, and then analyzed by Western blotting using anti-FLAG M2, anti-Myc 9E10, or anti-phosphotyrosine PY20. B, Co-immunoprecipitaion of Git1 mutants with Hic-5. The immunoprecipitates from cell extracts with anti-FLAG beads were analyzed by Western blotting with an anti-FLAG or anti-Myc antibody. To verify the tyrosine phosphorylation of FLAG-tagged Git1 proteins, the same membrane was reacted with anti-phosphotyrosine PY20. Ig, immunoglobulin. The lower part shows the densitometric analysis of the relative amount of Myc-Hic-5 to FLAG-Git1 in the immunoprecipitates. Data are the mean ± S.E. (error bars; n = 3). **, P < 0.01 significantly different from the wild-type with the same treatment; #, P < 0.05 or ##, P < 0.01 significant difference between vehicle- and pervanadate-treated groups by ANOVA with Fisher’s PLSD post hoc tests.
