Fig 10. A proposed model.
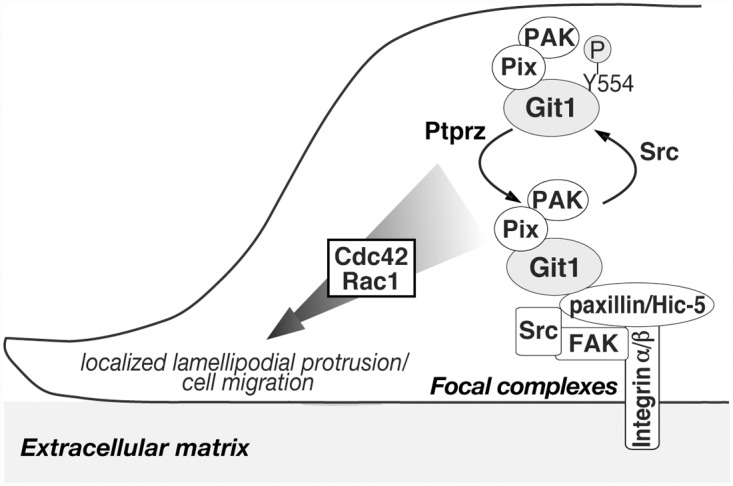
The Tyr-554 phosphorylation of Git1 is dependent on a Src tyrosine kinase [7, 12, 13]. The Git1-Pix complex dissociates from focal complexes by Tyr-554 phosphorylation, allowing the complex to recycle following dephosphorylation by tyrosine phosphatases, such as Ptprz [12, 13, 32]. Although FAK (focal adhesion kinase) is activated in response to integrin engagement, it is not yet clear whether FAK directly phosphorylates the Tyr-554 site. A stable complex of Git1-Pix containing PAK may reversibly associate with paxillin or Hic-5 at focal complexes by cyclic phosphorylation-dephosphorylation at Tyr-554, which is required to ensure the appropriate activation of Cdc42/Rac1 for localized protrusion activity in the front of the cells and co-ordinated cell movement: see also [4, 7–10, 35–41]. Here it should be noted that paxillin at Tyr-118 was also dephosphorylated by Ptprz [12]; however, the corresponding phosphorylation site is not present in Hic-5. The phosphorylated Tyr-118 and Tyr-31 residues reportedly serve as the binding sites for several src homology 2 (SH2) domain-containing proteins such as p120RasGAP and CrkII, and the phosphorylation of the two sites is necessary for efficient leading-edge protrusions during cell migration [42].
