Figure 6.
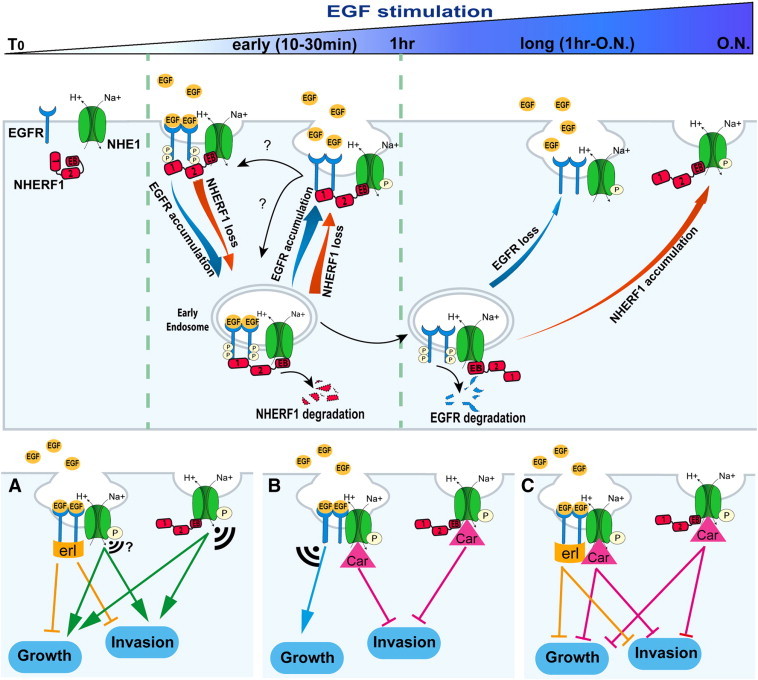
Mechanism of dynamics of the EGFR-NHERF1-NHE1 signaling axis and its role in PDAC growth and invasion.
In control cells EGFR, NHERF1, and NHE1 are separate. Short-term EGF stimulation (0-30 min) induces a transient, trimeric, EGFR-NHERF1-NHE1 complex, which segregates into two dimeric complexes (EGFR-NHE1 and NHERF1-NHE1) after prolonged EGF-stimulation. Upon EGF stimulation, NHERF1 expression decreases via proteosomal degradation, and EGFR is internalized and trafficked to intracellular vesicles by associating with the remaining NHERF1. From endosomes, a minor part of EGFR (≈ 40%), bound to NHERF1, is rapidly recycled back to the cell surface (30 minutes of EGF stimulation) where EGFR, NHERF1, and NHE1 are recruited together in a EGFR-NHERF1-NHE1 complex, whereas the remaining endosomal EGFR (≈ 60%) is destined to lysosomal degradation at longer EGF stimulation. This process avoids new cycles of plasma membrane (PM) receptor stimulation; permits an increase in the PM abundance of NHERF1, whose cellular expression is balanced by EGFR expression levels; and, importantly, favors the segregation of the trimer complex in two subcomplexes residing in specific regions of the PM where polarized NHE1 signaling occurs: the EGFR-NHE1 subcomplex is retained in caveolae and the NHERF1-NHE1 subcomplex in a different subset of PM invaginations. In this scenario, this branch point in the EGFR signaling pathway leading to NHE1 signal diversification and specificity may account for PDAC’s partial response to erlotinib and could explain why NHE1 inhibition with cariporide is important for combination therapy with erlotinib but also an important independent therapeutic approach in PDAC. Indeed, panels (A), (B), and (C) show the hypothetical effect of treatment with erlotinib (erl), 1 μM cariporide (Car), and both inhibitors, respectively, on long-term signaling and metastatic function. The size of the signal indicates the amplitude of the “residual” signaling from the different NHE1-containing subcomplexes.
