Figure 1.
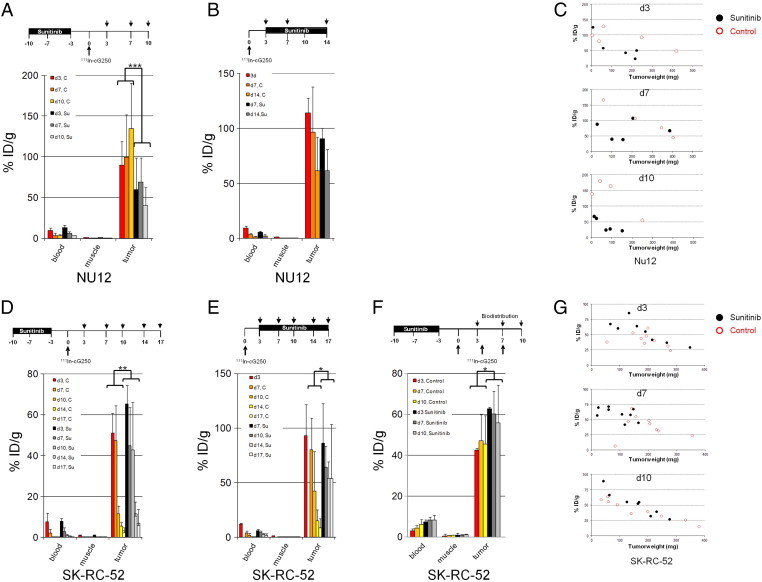
Biodistribution of cG250 in nude mice with NU12 and SK-RC-52 tumors.
111In-Girentuximab biodistribution in BALB/c nu/nu mice shows decreased uptake with sunitinib in NU12 tumors and increased uptake with sunitinib in SK-RC-52 tumors. Treatment schedules shown on top of graphs.
Groups of 4–5 mice (in F: 8–9 mice) were treated with sunitinib every day for 1 week (A,D,F) or until they were euthanized (B,E). Three days before start or 3d (in one experiment also 7 or 10 days) after stop of treatment, mice were injected with 111In-Girentuximab (0.4 MBq, 5 μg) and mice were euthanized at various timepoints. The activity in the samples was expressed as % injected dose per gram tissue (%ID/g). A: Biodistribution of NU12 mice with sunitinib treatment preceding 111In-Girentuximab injection, B: Biodistribution of NU12 mice injected with 111In-Girentuximab before sunitinib treatment, C: G250 antibody uptake was plotted for individual tumors from experiment. Red open circles: control tumors; black closed circles: sunitinib treated tumors A, D: Biodistribution of SK-RC-52 mice treated with sunitinib preceding 111In-Girentuximab injection, E: Biodistribution of SK-RC-52 mice treated with sunitinib followed by 111In-Girentuximab, F: Biodistribution of SK-RC-52 mice with 111In-Girentuximab injection 3 days, 7 days or 10 days after cessation of sunitinib, G: G250 antibody uptake was plotted for individual tumors from experiment F. *P < .05, **P < .01, ***P < .005. P-values shown for the biodistribution are based on all sunitinib treated animals (N = 15, 9, 28,18, 25 for Figure 1, A, B, D–F, respectively) compared to all control animals (N = 13, 16, 30, 18, 27 for Figure 1, A, B, D–F, respectively).
