Abstract
Human thymus is completely developed in late fetal stages and its function peaks in newborns. After the first year of life, the thymus undergoes a progressive atrophy that dramatically decreases de novo T-lymphocyte maturation. Hormonal signaling and changes in the microRNA expression network are identified as underlying causes of human thymus involution. However, specific pathways involved in the age-related loss of thymic function remain unknown. In this study, we analyzed differential gene-expression profile and microRNA expression in elderly (70 years old) and young (less than 10 months old and 11 years old) human thymic samples. Our data have shown that WNT pathway deregulation through the overexpression of different inhibitors by the nonadipocytic component of the human thymus stimulates the age-related involution. These results are of particular interest because interference of WNT signaling has been demonstrated in both animal models and in vitro studies, with the three major hallmarks of thymic involution: (i) epithelial structure disruption, (ii) adipogenic process, and (iii) thymocyte development arrest. Thus, our results suggest that secreted inhibitors of the WNT pathway could be explored as a novel therapeutical target in the reversal of the age-related thymic involution.
Key Words: Human thymus, Thymus involution, WNT pathway, Aging.
The human thymus is the major site of T-cell development (1) and, in consequence, one of the main organs involved in the generation and maintenance of the adaptive immune system. The thymus comprises a hematopoietic compartment of developing thymocytes that progress through a series of distinct developmental stages under the instructive action of the supporting stromal microenvironment. These complex maturation and selection processes allow the generation of functional, but not self-reacting, T lymphocytes. The thymus becomes fully functional during fetal life and peaks in newborns, whereupon it undergoes a progressive atrophy that dramatically decreases the T-lymphocyte generation (2,3).
Thymic involution is characterized by a depletion of developing thymocytes as well as the supporting thymic epithelial cells (TECs), leading to a loss of thymic architecture and decrease in its function (2,4). In the aging human thymus, adipose tissue infiltrates the disorganized epithelial mesh; however, the origin of these adipocytes remains unclear. Ultimately, the ability of the thymus to support optimal thymocyte development is considerably reduced with age, and immature thymocytes are arrested at the double-negative stage (1–4). Several underlying causes that can affect on age-related thymic involution have been identified, including (i) growth hormone receptor expression by TECs and the production of its cognate ligand (5,6); (ii) increased production of sex steroids (2) along with androgen blockade reported to enhance thymus function in both mice and humans (7); (iii) glucocorticoids, which, along with other hormones of the hypothalamic-pituitary-adrenal axis decrease thymus functionality (8), primarily by inducing IL-6 production (9,10); (iv) the catecholamine network, which has been reported as an important modulator of thymic function (11); and (v) the microRNA (miRNA) network, which has recently been described as a novel pathway involved in the control of thymic involution (12). However, despite the considerable advances in understanding the primary causes of thymic involution, the specific pathways involved in the atrophy of the thymus, in both mice and humans, remain unclear.
Thus, the aim of this study was to analyze gene expression and changes to miRNAs in thymus samples derived from young or old human participants.
Materials and methods
Thymus Samples
Thymus samples from adult and elderly male individuals, aged between 50 and 70 years, who underwent cardiac surgery (valvular repair or ischemic cardiopathy) at Virgen del Rocio University Hospital in Seville, Spain, were obtained. Thymus samples from 10-month-old newborns to 11-year-old children were also obtained after cardiac surgery procedures (congenital heart diseases). Individuals who were diagnosed of Down syndrome were excluded from the study. None of the donors had received any treatment that could influence their immune status and did not show clinical data of active infections, including an HIV-negative test. Aged thymus remained clearly distinguishable from that found in the adipose tissue of the mediastinum, as previously reported (13).
Samples committed to the array analysis were frozen and preserved for further use (n = 4 per group); otherwise, fresh samples were processed immediately (n = 4 for the young group and n = 3 in the adulthood and elderly groups). Fresh thymic samples were washed twice with phosphate-buffered saline and then DNase I (Life Technologies, Grand Island, NY) and collagenase D (Roche Applied Science, Indianapolis, IN) digestion (45 min at 37°C with gentle shaking) is carried out, with moderate mechanical disruption. A last digestion step (30 min at 37°C with gentle shaking) using a collagenase/dispase mixture (Roche Applied Science) was performed to ensure complete tissue disaggregation. After a brief spin (30 s, 300 g), adipocytes were carefully aspirated from the supernatant. Isolated adipocytes and the remaining adipocyte-free thymic tissue were washed twice using phosphate-buffered saline and then prepared for RNA extraction.
Participants, or their legal caregivers, were informed of and provided with the written consent to participate in the study. The Ethical Committee of the Virgen del Rocio Hospital in Seville, Spain, approved the study. Thymic tissue samples were always obtained for clinical reasons but never for research purposes.
Gene Expression Profiling
The experiment was performed using Illumina HumanRef-8 v3 array, which analyzes 245,333 human transcripts. The objective of the analysis was to compare gene expression profiling from thymic samples of young versus adult. Microarray data were analyzed using Genespring software (Agilent Technologies, Santa Clara, CA). After data normalization, genes with low-quality signals were excluded from statistical analysis. To detect differentially expressed genes, we performed a t test with false discovery rate control, which is estimated using a single-step Bonferroni procedure. A gene was considered differentially expressed if the corrected p value for multiple testing was less than .05. Among the genes that passed the t test filter, only genes showing a mean log ratio value lower than −0.3 or greater than 0.3 (equivalent to a twofold change) were selected as differentially expressed. Heat maps were generated using the Babelomics 4.0 online software, as previously reported (14).
miRNA Expression Profiling
Locked nucleic acid-miRNA microarrays were performed using the Agilent Human microRNA V2.0 array, which was used to analyze 723 human and 76 human viral miRNAs (799 total miRNAs). The miRNA data were processed with the “limma” Bioconductor package in the R statistical environment. Although differential expression was sensitive to normalization choices, we identified miRNA candidates that were robustly observed across a variety of methods that use rank product analysis. The biological sense of the results was confirmed by testing the identified hsa-miRNAs for known organ specificity, previous implication in aging, and compatibility with pathways identified through messenger RNA (mRNA) microarray analysis.
Relative Quantitative Polymerase Chain Reaction Assay
Quantitative polymerase chain reaction (qPCR) standard procedures were used to verify the mRNA array results. Briefly, mRNA-related cDNA was synthesized from TRIzol-treated (Life technologies, Grand Island, NY) thymic samples (complete tissue, adipocytes, and adipocyte-free samples), using the SuperScript III Cells Direct cDNA synthesis system (Life technologies) and polyA primers, according to the manufacturers’ instructions. Using 400ng of cDNA, 375nm of each primer, and 1× LC480 SYBR Green I Master buffer (Roche Applied Science), the qPCR was performed in a LightCycler 480 (Roche Applied Science). PCR conditions were as follows: a first denaturation step (10 min) was followed by 50 cycles for 15 s at 95°C, 15 s at 56°C, and 15 s at 72°C. To ensure the specificity of the obtained data, a final melting curve analysis was performed. All primer sets are detailed in Supplementary Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and hypoxanthine-guanine phosphoribosyl transferase (HPRT) were used as reporter genes. Each PCR plate was designed to analyze six different genes, with samples from three young and three old individuals. For every analyzed gene, standard curves were created and used as external curves to correct the qPCR efficiency.
Results
mRNA Expression Microarray Analysis
Thymic tissue samples obtained from four old (70-year-old men) and four young (<10-month-old newborns) individuals were analyzed in a cDNA-based microarray to determine the transcriptional changes associated with thymic involution. Results showed that from a total of 24,533 genes, 1,606 genes were altered by greater than twofold (p < .05) with age. Of these genes, 658 were downregulated and 948 were upregulated in the thymus samples from old individuals compared with those from young individuals. Fold change values from genes differentially expressed in elderly thymic samples are shown in Figure 1. We then analyzed alteration in pathways biologically relevant for thymic involution. We found significant alterations in the canonical WNT–β-catenin pathway, which was of particular interest, considering that disruption of WNT signaling has been implicated in impairment of thymocyte development (15,16), TEC structure disorganization (17), and adipogenic processes (18)—all of which are also associated with the aging thymus.
Figure 1.
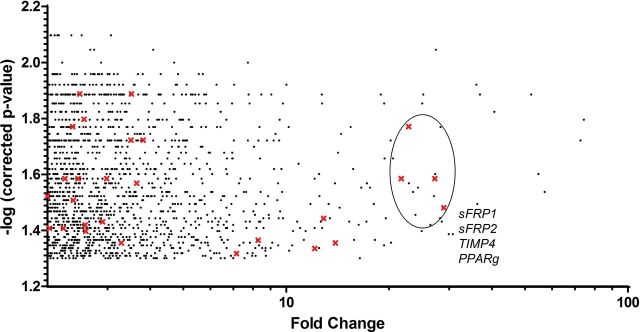
Fold change versus corrected p value (by a false discovery rate–corrected threshold of 0.05) from all age-related deregulated genes. WNT pathway genes are highlighted with crosses. From them, the sFRP extracellular inhibitors and the TIMP4 cytoplasmatic inhibitor are the most significantly deregulated (upregulated). PPARγ was also strongly upregulated.
WNT signaling stabilizes β-catenin, allowing its translocation to the nucleus and the activation of downstream transcription factors. Table 1 summarizes all WNT-related genes that were altered in our analysis. Although we observed increased expression of WNT receptor components, including FZD4 (WNT receptor), LRP3 (WNT coreceptor), and GBP proteins (part of the β-catenin stabilization complex), inhibitors of downstream WNT signals were strongly increased (Figure 2A and B). Consequently, WNT-related transcription factor expression was deregulated in old thymuses (Figure 2A and B). We also observed a decrease in axin expression, which is a conventional marker of WNT pathway function. Together, these findings illustrated that all levels of the WNT pathway (extracellular, cytosolic, and intranuclear) were inhibited in the old thymic tissue, demonstrating a profound arresting of WNT signaling in the senescent thymus. In addition to these changes in WNT signaling, PPARγ and C/EBPα transcription factors, both required for adipogenesis, were also increased (Figure 2A and B).
Table 1.
Overview of WNT-Related Altered Genes
| Gene | Accession Number | Up/down | Fold Change | Location |
|---|---|---|---|---|
| sFRP1 | NM_00301.3 | Up | 28.72 | Extracellular membrane |
| sFRP2 | NM_003013.2 | Up | 22.68 | |
| FrzB | NM_001463.2 | Up | 02.49 | |
| HEX | NM_002729.4 | Up | 03.51 | |
| FZD4 | NM_012193.2 | Up | 12.80 | |
| LRP3 | NM_002333.1 | Up | 08.25 | |
| GBP2 | NM_004120.3 | Up | 02.90 | Cytoplasmatic |
| GBP4 | NM_052941.3 | Up | 02.58 | |
| Axin2 | NM_004655.2 | Down | 02.00 | |
| TIMP1 | NM_003254.2 | Up | 02.38 | |
| TIMP3 | NM_000362.4 | Up | 13.85 | |
| TIMP4 | NM_003256.2 | Up | 21.58 | |
| CTNNA1 | NM_001903.2 | Up | 03.29 | |
| TLE2 | NM_003260.3 | Up | 02.46 | Nuclear inhibitors |
| TLE4 | NM_007005.3 | Down | 02.23 | |
| FOXO1 | NM_002015.3 | Up | 02.98 | |
| TCF7L1 | NM_031283.1 | Up | 02.59 | |
| TCF7L2 | NM_003199.2 | Up | 02.37 | |
| FOXM1 | NM_202003.1 | Down | 03.65 | β-catenin stabilization |
| TCF7 | NM_213648.1 | Down | 07.13 | Nuclear – transcription factors |
| TCF7 | NM_201634.1 | Down | 03.81 | |
| TCF7 | NM_201633.1 | Down | 02.56 | |
| TCF7 | NM_003202.2 | Down | 02.03 | |
| TCF12 | NM_207037.1 | Down | 03.52 | |
| TCF19 | NM_001077511.1 | Down | 02.25 | |
| PPARγ | NM_015869.4 | Up | 27.03 | Adipogenesis |
| C/EBPα | NM_004364.2 | Up | 12.60 |
Note: Bolded genes showed a fold change >5.
Figure 2.
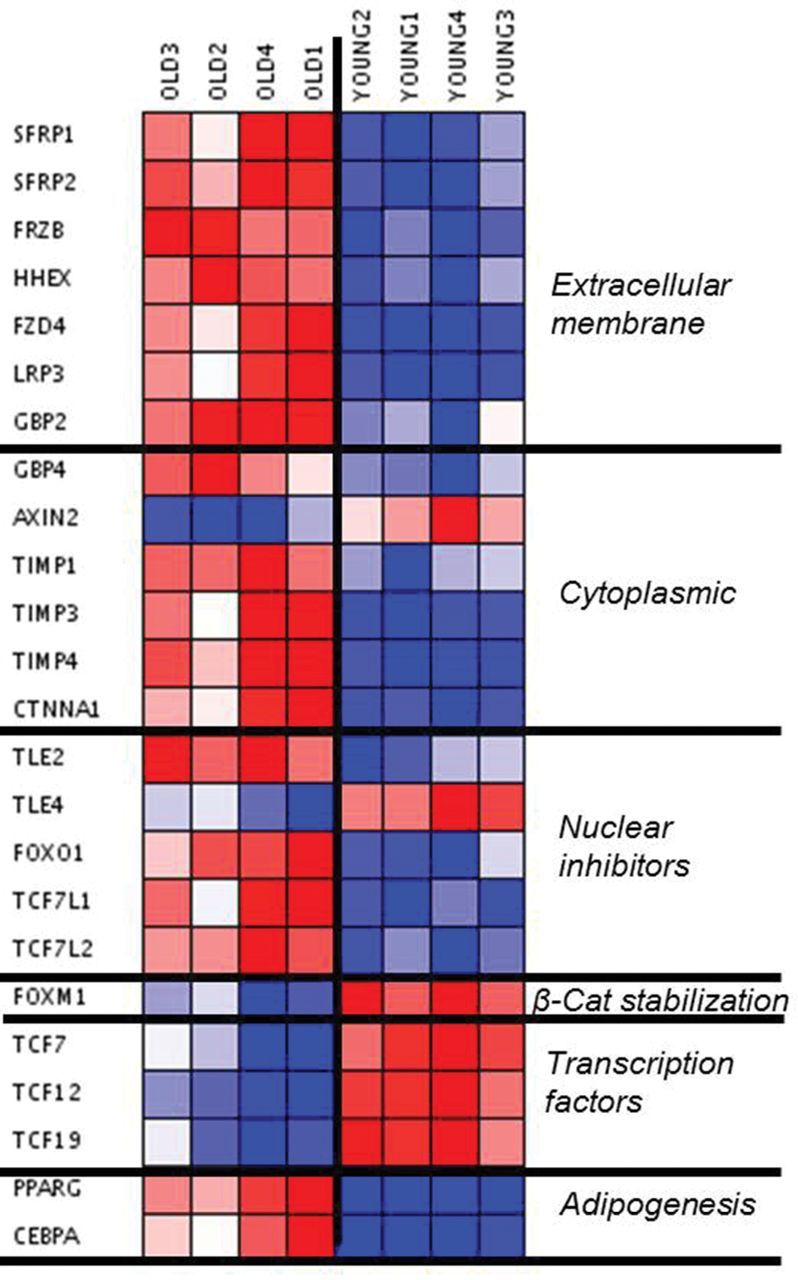
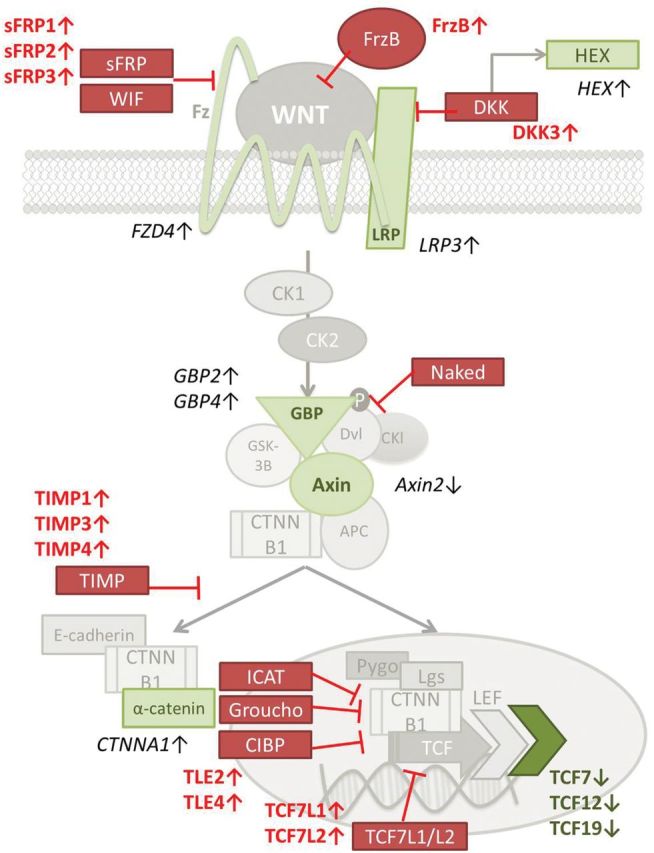
(A) Visual representation (heat map) of WNT-related altered genes. Downregulated = blue. Upregulated = red. (B) Schematic representation of the WNT pathway. The results of the microarray analysis showed the red-marked WNT mediators as upregulated, whereas green-marked WNT mediators were found downregulated, in involuted (elderly) thymus samples. Four thymic tissue samples were analyzed for each group.
qPCR Validation
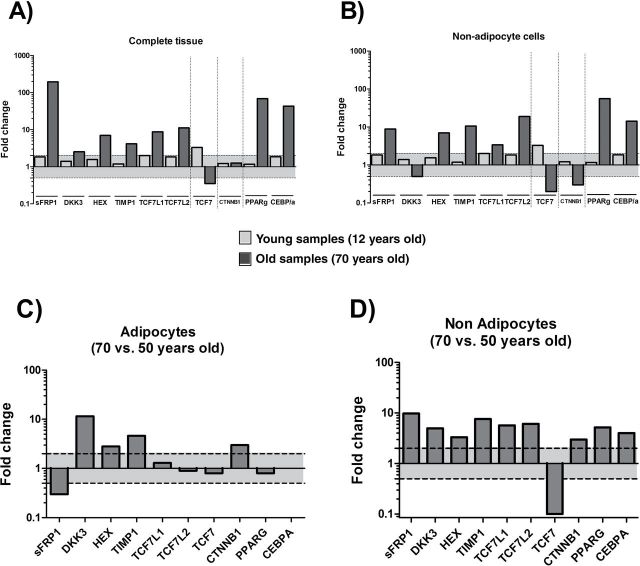
To confirm and verify the microarray results, we performed qPCR analysis on selected inhibitors representing all levels of the WNT pathway (Figure 2). In accordance with our microarray analysis, PPARγ, C/EBPα, and sFRP1 were significantly upregulated, demonstrating up to 100-fold change with age (Figure 3A, dark gray bars). HEX, TIMP1, TCF7L1/L2, and DKK3 were also increased, whereas TCF7 gene expression was decreased. However, WNT pathway signaling is crucial for key processes during human development (19). Thus, even if we were analyzing samples from individuals before their first year of age (10-month-old group), which thymus is completely functional, we wanted to confirm whether differences observed between 10-month-old and 70-year-old thymic tissues were actually related with the involution process. Thus, we analyzed thymic tissue samples for gene expression in a group of 11-year-old children. As shown in Figure 3A (light gray bars), the WNT pathway is similarly regulated in the 10-month-old and 11-year-old groups, thus strongly suggesting that the increase of inhibitors in the WNT pathway in elderly thymus samples is mainly related with the involution processes.
Figure 3.
Quantitative polymerase chain reaction validation. Young (11 years old, n = 4)—light gray bars—and old (70 years old, n = 3)—dark gray bars—expression patterns compared with 10-month-old infants in (A) complete human thymic tissue and (B) adipocyte-depleted human thymic tissue. (C) Old (70 years old, n = 3) versus middle-aged (50 years old, n = 3) human thymus–derived adipocytes. (D) Adipocyte-depleted old (70 years old, n = 3) versus adipocyte-depleted middle-aged (50 years old, n = 3) human thymic tissue. In all figures, the log ratio value of ±0.3 (equivalent to a twofold change) cutoff value is showed as a sensitivity limit of differences between groups.
Given the considerable cellular alterations in the thymus with age, we could not exclude that the observed differences in both, microarray and qPCR analyses, reflect changes in subset composition, rather than true age-related changes in gene expression. To account for the significant age-related differences in the contribution of adipocytes to overall thymus cellular content, we reanalyzed thymic tissue derived from 70-year-old individuals, following the removal of all adipocytes. In line with our results, we found that individuals from both 10-month-old group (Figure 3B, light gray bars) and 11-year-old group show similar expression levels of WNT-related genes. In addition, the WNT pathway inhibition is also observed (Figure 3B, dark gray bars) even when true thymic tissue samples, after removing the adipocytic component, were to be analyzed.
For better understanding the role of adipocytic and nonadipocytic tissues in the WNT pathway regulation, we analyzed gene expression of both cell types in adult thymic samples (50-year-old individuals) versus atrophied samples (70-year-old individuals). Adulthood age was chosen for the study because the age of 50 years has been shown as a critical impasse age for thymic function (13). Similar expression levels of nuclear inhibitors (TCF7L1/L2), TCF7, PPARγ, and C/EBPα were found in the adipocyte fraction (Figure 3C) among the age groups. However, on assessing the contribution of nonadipocytic cells (mainly TECs and thymocytes), we found that all inhibitors were upregulated (Figure 3D). Increased expression of WNT inhibitors was simultaneously observed, together with a strong downregulation of the TCF7 transcription factor in the thymus samples from 70-year-old individuals. Interestingly, PPARγ and C/EBPα were also upregulated in 70-year-old adipocyte-free thymic tissue (compared with 50-year-old counterparts).
miRNA Expression Microarray Analysis
In addition, we also assessed the difference in miRNA expression between old (70-year-old men) and young thymus (<10-month-old newborns). Out of 799 miRNAs, 106 were significantly changed in the elderly thymus. From these, 78 miRNAs were downregulated and 28 were upregulated (Table 2). Importantly, members of the miR-17–92 cluster, which have been previously related to aging and cancer processes (20), were downregulated in senescent thymus samples. In addition, several other miRNAs exhibited differential expression in the senescent thymus, including miR-22, miR-15a, miR-16, and the miR-181 family, all of which have previously been implicated in thymus involution caused by aging (21) or stress (22). Besides, in line with our findings on mRNA, miRNAs that have been reported as modulators of the WNT pathway, including miR-25, miR-7f, and miR-134, were also found altered (23). Finally, miRNAs of the miR-200 cluster (24,25), which have an active role in blocking the epithelial to mesenchymal transition processes, were found downregulated in the elderly thymus. These results, together with the increased expression of PPARγ and C/EBPα transcription factors in nonadipocytic tissue (Figure 3D), suggest that the increase of intrathymic adipocytes with age could be generated from senescent TECs through epithelial to mesenchymal transition processes.
Table 2.
Overview of the h-miRNA Expression Modified in Elderly Tissue
| h-miR Number | Differential Expression | Previously Described Role | |
|---|---|---|---|
| miR-17 | −1.0 | miR-17–92 cluster (aging and cancer) | Replicative + organismal human aging |
| miR-18a | −2.1 | ||
| miR-19a | −1.7 | Organismal human aging | |
| miR-19b | −1.2 | Replicative + organismal human aging | |
| miR-20a | −2.1 | Replicative + organismal human aging | |
| miR-20b | −2.3 | Organismal human aging | |
| miR-25 | −1.7 | WNT modulator | |
| miR-92a | −0.9 | ||
| miR-93 | −1.3 | ||
| miR-106a | −2.3 | Replicative + organismal human aging | |
| miR-106b | −1.3 | ||
| miR-363 | −3.0 | ||
| miR-7i | −0.9 | Organismal human aging | |
| miR-7f | −1.1 | WNT modulator | |
| miR-15a | −1.8 | ||
| miR-16 | −1.9 | ||
| miR-146a | −1.0 | ||
| miR-193b | 0.6 | ||
| miR-335 | 1.2 | WNT modulator | |
| miR-424 | −1.8 | ||
| miR-22 | 1.1 | Replicative human aging | |
| miR-128 | −2.8 | Stress-related thymic involution | |
| miR-181a | −2.4 | ||
| miR-181a-2 | −1.5 | ||
| miR-181b | −1.8 | ||
| miR-200a | −1.3 | Epithelial to mesenchymal transition | |
| miR-200b | −1.5 | ||
| miR-200c | −1.3 | ||
| miR-134 | 0.5 | WNT modulator | |
| miR-223 | −1.2 |
Discussion
Our study shows that WNT signaling is downregulated in the involuted human thymus. These results suggest that the canonical WNT pathway could have an active role in the thymus atrophy process because inhibition of its signaling is compatible with the three hallmark processes of thymic involution: arrest of thymocyte differentiation, loss of the corticomedular organization of TECs, and epithelial-derived differentiation of adipocytes.
Several studies have demonstrated that intrathymic double-negative to double-positive thymocyte maturation is dependent on a WNT-induced transitory stabilization of β-catenin (15,16,26). WNT proteins, which are necessary for this process, are primarily produced by TECs (27). In mice models, premature thymic involution is induced upon deregulation of WNT signaling (17), and WNT pathway alterations in the mouse pharyngeal mesenchyme during development cause a DiGeorge-like phenotype (28). Given these findings, it is not surprising that maintenance of the thymic epithelia requires WNT signaling (29), and diminished expression of WNT proteins—or increased levels of WNT inhibitors—is associated with TEC senescence (30). These findings are consistent with the increase of WNT inhibitors, targeting numerous levels of WNT signaling, in involuted human thymic samples.
An appealing result is that, regarding the WNT pathway, thymic tissue samples from both 11-year-old group and 10-month-old group showed similar gene expression patterns. Thymic tissue involution has been reported to start as early as in the first year of life, whereas a second wave of tissue involution occurs, coinciding with pubertal changes (2–4). Thymic involution is faster after this second wave. According to our results, WNT pathway deregulation does not occur in prepubertal (11 years old) participants, strongly suggesting that alterations in the WNT signaling are part of this second—and faster—wave of thymic involution.
Diminished secretion of WNT proteins by TECs increases PPARγ expression, promoting epithelial to mesenchymal transition processes (31). Consistent with this finding, mesodermal cells avoid entering an adipogenic program by secreting WNT proteins (18), and disruption of WNT signaling results in spontaneous adipogenesis (32). PPARγ expression in thymic tissue has been reported to increase with age and could be associated with decreased peripheral naive T-cell numbers and contraction of TCR diversity (33). Caloric restriction—the most effective method to slow down aging in animal models—avoids PPARγ-related adipocyte generation (34,35). In agreement, our results suggest that the TEC and/or thymocytes from elderly thymi, through WNT inhibition, are stimulation strongly stimulating adipogenesis. These findings show that WNT pathway inhibition as a possible mechanism of human thymic involution. In addition, despite further validation is still needed, miRNA alterations showed in our study are fully compatible a strongly deregulated WNT pathway in the involuted thymic tissue.
Finally, several systemic factors can induce thymic involution: the hypothalamic-pituitary-adrenal-gonadotropic axis (36) with cortisol, catecholamine (11), androgens (7), growth hormone/ghrelin (5,6), and corticosteroids (9,10) is capable of modulating thymic function. Interestingly, despite different absolute effects and mechanisms, WNT signaling is a prominent mediator for growth hormone (37), IGF-1 (38), androgens (39,40), and among others (41,42).
Altogether, our results provide evidence for an important role of the WNT signaling inhibition in the human thymus involution process. Moreover, these studies suggest that modulating the secretion of WNT pathway inhibitors could be an interesting target for reversing the atrophy process and boosting adulthood thymic function.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
S.F.-M. and E.R.-M. have grants from the Fondo de Investigaciones Sanitarias (CD10/00382 and CP08/00172, respectively). J.G. is supported by FWF (P 24498-B20), GENAU project 820982 “Non-coding RNAs,” the Herzfelder’sche Familienstiftung, and Ce.R.I.E.S. This project has been supported by ISCIII RETIC RD12/0017/0029 and RD12/0017/0037, Proyecto de Excelencia, CICE (CTS-6313), and Consejería de Salud (PI-0278-2010). This research was supported by National Institutes of Health award numbers R01-HL069929 (M.R.M.v.d.B.), R01-CA107096 (M.R.M.v.d.B.), R01-AI080455 (M.R.M.v.d.B.), and P01-CA023766 (ROR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support was also received from the U.S. Department of Defense: USAMRAA Award W81XWH-09-1-0294 (M.R.M.v.d.B.), the Radiation Effects Research Foundation (RERF-NIAID; M.R.M.v.d.B.), The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by Mr. William H. Goodwin and Mrs. Alice Goodwin, The Lymphoma Foundation, Alex’s Lemonade Stand, The Geoffrey Beene Cancer Research Center at Memorial Sloan-Kettering Cancer Center, and The Peter Solomon Fund.
Supplementary Material
Acknowledgments
Authors are very grateful to all donors and also to the cardiac surgery team of the Virgen del Rocio University Hospital. We also thank Marta de Luna Romero for excellent technical assistance.
References
- 1. Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. :10.1146/annurev.immunol.18.1.529 [DOI] [PubMed] [Google Scholar]
- 2. Chiodi H. The relationship between the thymus and the sexual organs. Endocrinology. 1940;26:107–116. http://dx.doi.org/10.1210/endo- 26-1-107 [Google Scholar]
- 3. Steinmann GG. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. [DOI] [PubMed] [Google Scholar]
- 4. Henry L. Involution of the human thymus. J Pathol Bacteriol. 1967;93:661–671. :10.1002/path.1700930227 [DOI] [PubMed] [Google Scholar]
- 5. Welniak LA, Sun R, Murphy WJ. The role of growth hormone in T-cell development and reconstitution. J Leukoc Biol. 2002;71:381–387. :10.1189/jlb.1938-3673 [PubMed] [Google Scholar]
- 6. Taub DD, Murphy WJ, Longo DL. Rejuvenation of the aging thymus: growth hormone-mediated and ghrelin-mediated signaling pathways. Curr Opin Pharmacol. 2010;10:408–424. :10.1016/j.coph.2010.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sutherland JS, Goldberg GL, Hammett MV, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. [DOI] [PubMed] [Google Scholar]
- 8. de Felice C, Toti P, Musarò M, et al. Early activation of the hypothalamic-pituitary-adrenal-axis in very-low-birth-weight infants with small thymus at birth. J Matern Fetal Neonatal Med. 2008;21:251–254. :10.1080/14767050801927871 [DOI] [PubMed] [Google Scholar]
- 9. Sempowski GD, Hale LP, Sundy JS, et al. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J Immunol. 2000;164:2180–2187. [DOI] [PubMed] [Google Scholar]
- 10. Sempowski GD, Rhein ME, Scearce RM, Haynes BF. Leukemia inhibitory factor is a mediator of Escherichia coli lipopolysaccharide-induced acute thymic atrophy. Eur J Immunol. 2002;32:3066–3070. :10.1002/1521-4141(200211)32:11<3066::AID-IMMU3066>3.0.CO;2-J [DOI] [PubMed] [Google Scholar]
- 11. Leposavić G, Pilipović I, Perišić M. Age-associated remodeling of neural and nonneural thymic catecholaminergic network affects thymopoietic productivity. Neuroimmunomodulation. 2012;18:290–308. :10.1159/000329499 [DOI] [PubMed] [Google Scholar]
- 12. Papadopoulou AS, Dooley J, Linterman MA, et al. The thymic epithelial microRNA network elevates the threshold for infection-associated thymic involution via miR-29a mediated suppression of the IFN-α. Nat Immunol. 2011;13:181–187. :10.1038/ni.2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrando-Martínez S, Franco JM, Hernandez A, et al. Thymopoiesis in elderly human is associated with systemic inflammatory status. Age (Dordr). 2009;31:87–97. :10.1007/s11357-008-9084-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Medina I, Carbonell J, Pulido L, et al. Babelomics: an integrative platform for the analysis of transcriptomics, proteomics and genomic data with advanced functional profiling. Nucleic Acids Res. 2010;38:W210–W213 doi:10.1093/nar/gkq388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu M, Sharma A, Hossain MZ, Wiest DL, Sen JM. Sustained expression of pre-TCR induced beta-catenin in post-beta-selection thymocytes blocks T cell development. J Immunol. 2009;182:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu Q, Sharma A, Sen JM. TCF1 and beta-catenin regulate T cell development and function. Immunol Res. 2010;47:45–55. :10.1007/s12026-009-8137-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zuklys S, Gill J, Keller MP, et al. Stabilized beta-catenin in thymic epithelial cells blocks thymus development and function. J Immunol. 2009;182:2997–3007. :10.4049/jimmunol.0713723 [DOI] [PubMed] [Google Scholar]
- 18. Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. :10.1126/science.289.5481.950 [DOI] [PubMed] [Google Scholar]
- 19. van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. :10.1242/dev.033910 [DOI] [PubMed] [Google Scholar]
- 20. Grillari J, Hackl M, Grillari-Voglauer R. miR-17–92 cluster: ups and downs in cancer and aging. Biogerontology. 2010;11:501–506. :10.1007/s10522-010-9272-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hackl M, Brunner S, Fortschegger K, et al. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell. 2010;9:291–296. :10.1111/j.1474-9726.2010.00549.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belkaya S, Silge RL, Hoover AR, et al. Dynamic modulation of thymic microRNAs in response to stress. PLoS One. 2011;6:e27580. :10.1371/journal.pone.0027580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anton R, Chatterjee SS, Simundza J, Cowin P, Dasgupta R. A systematic screen for micro-RNAs regulating the canonical Wnt pathway. PLoS One. 2011;6:e26257. :10.1371/journal.pone.0026257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mongroo PS, Rustgi AK. The role of the miR-200 family in epithelial-mesenchymal transition. Cancer Biol Ther. 2010;10:219–222. :10.4161/cbt.10.3.12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiong M, Jiang L, Zhou Y, et al. The miR-200 family regulates TGF-β1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J Physiol Renal Physiol. 2012;302:F369–F379. :10.1152/ajprenal.00268.2011 [DOI] [PubMed] [Google Scholar]
- 26. Valencia J, Hernández-López C, Martínez VG, et al. Transient beta-catenin stabilization modifies lineage output from human thymic CD34+CD1a- progenitors. J Leukoc Biol. 2010;87:405–414. :10.1189/jlb.0509344 [DOI] [PubMed] [Google Scholar]
- 27. Weerkamp F, Baert MR, Naber BA, et al. Wnt signaling in the thymus is regulated by differential expression of intracellular signaling molecules. Proc Natl Acad Sci U S A. 2006;103:3322–3326. :10.1073/pnas.0511299103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huh SH, Ornitz DM. Beta-catenin deficiency causes DiGeorge syndrome-like phenotypes through regulation of Tbx1. Development. 2010;137:1137–1147. :10.1242/dev.045534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Osada M, Jardine L, Misir R, Andl T, Millar SE, Pezzano M. DKK1 mediated inhibition of Wnt signaling in postnatal mice leads to loss of TEC progenitors and thymic degeneration. PLoS One. 2010;5:e9062. :10.1371/journal.pone.0009062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Varecza Z, Kvell K, Talabér G, et al. Multiple suppression pathways of canonical Wnt signalling control thymic epithelial senescence. Mech Ageing Dev. 2011;132:249–256. :10.1016/j.mad.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kvell K, Varecza Z, Bartis D, et al. Wnt4 and LAP2alpha as pacemakers of thymic epithelial senescence. PLoS One. 2010;5:e10701. :10.1371/journal.pone.0010701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bennett CN, Ross SE, Longo KA, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–31004. :10.1074/jbc.M204527200 [DOI] [PubMed] [Google Scholar]
- 33. Youm YH, Yang H, Amin R, Smith SR, Leff T, Dixit VD. Thiazolidinedione treatment and constitutive-PPARgamma activation induces ectopic adipogenesis and promotes age-related thymic involution. Aging Cell. 2010;9:478–489. :10.1111/j.1474-9726.2010.00574.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang H, Youm YH, Dixit VD. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol. 2009;183:3040–3052. :10.4049/jimmunol.0900562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitterberger MC, Zwerschke W. Mechanisms of resveratrol-induced inhibition of clonal expansion and terminal adipogenic differentiation in 3T3-L1 preadipocytes. J Gerontol A Biol Sci Med Sci. 2013;68:1356–1376. :10.1093/gerona/glt019 [DOI] [PubMed] [Google Scholar]
- 36. Savino W, Arzt E, Dardenne M. Immunoneuroendocrine connectivity: the paradigm of the thymus-hypothalamus/pituitary axis. Neuroimmunomodulation. 1999;6:126–136. [DOI] [PubMed] [Google Scholar]
- 37. Miyakoshi T, Kajiya H, Miyajima K, et al. The expression of Wnt4 is regulated by estrogen via an estrogen receptor alpha-dependent pathway in rat pituitary growth hormone-producing cells. Acta Histochem Cytochem. 2009;42:205–213. :10.1267/ahc.09033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Novosyadlyy R, Leroith D. Insulin-like growth factors and insulin: at the crossroad between tumor development and longevity. J Gerontol A Biol Sci Med Sci. 2012;67:640–651. :10.1093/gerona/gls065 [DOI] [PubMed] [Google Scholar]
- 39. Warr N, Siggers P, Bogani D, et al. Sfrp1 and Sfrp2 are required for normal male sexual development in mice. Dev Biol. 2009;326:273–284. :10.1016/j.ydbio.2008.11.023 [DOI] [PubMed] [Google Scholar]
- 40. Shin YK, Cong WN, Cai H, et al. Age-related changes in mouse taste bud morphology, hormone expression, and taste responsivity. J Gerontol A Biol Sci Med Sci. 2012;67:336–344. :10.1093/gerona/glr192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blakely BD, Bye CR, Fernando CV, et al. Wnt5a regulates midbrain dopaminergic axon growth and guidance. PLoS One. 2011;6:e18373. :10.1371/journal.pone.0018373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van der Horst PH, Wang Y, Vandenput I, et al. Progesterone inhibits epithelial-to-mesenchymal transition in endometrial cancer. PLoS One. 2012;7:e30840. :10.1371/journal.pone.0030840 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.